2. 111In-Pentetreotide
Functional imaging studies based on the expression of SSTRs play an important role in evaluating patients with suspected GEP-NENs and high density of SSTRs. CT and MRI allow for anatomical characterization, but they cannot provide accurate functional information and are often insufficient for the diagnosis of GEP-NENs if the lesions are small and cover various anatomical locations. Somatostatin receptor scintigraphy (SRS) can provide important functional data. Thus, SRS shows good accuracy for whole-body imaging and has been routinely used for the diagnosis and follow-up of GEP-NENs [
17].
SRS using the somatostatin analog
111In-pentetreotide (
111In-DTPA-D-Phe-conjugate of octreotide; Octreoscan
®) was the first radiotracer agent to be approved for NENs in 1989 [
18]. The radiotracer preferentially binds to SSTR subtypes 2, 3 and 5, especially 2, and has proven to be an essential diagnostic functional imaging for the diagnosis, staging and follow-up of NENs, both pulmonary and, in particular, GEP-NENs. The added value of a whole body assessment in one setting is particularly advantageous.
111In-Pentetreotide scans are routinely obtained at 4 and 24 h after the intravenous administration of the radiotracer, and image acquisition is ideally performed with a double-headed gamma camera equipped with a medium-energy collimator [
18]. However, in spite of the advantage over CI, the principal limitation of using
111In-Pentetreotide is the low spatial resolution of the gamma camera that significantly affects the assessment of small lesions, particularly in organs with a high physiological uptake (such as the liver). The use of SPECT/CT imaging has markedly improved the accuracy of
111In-Pentetreotide, increasing its spatial resolution and the anatomic localization of pathologic sites of increased uptake due to CT co-registration, with decreased false positive results and its capacity to provide cross-sectional scintigraphic images [
19,
20,
21,
22,
23].
111In-Pentetreotide, acquired as SPECT/CT, shows higher sensitivity and accuracy values than CI and can be still used for routine diagnostic imaging of non-functioning NEN-GEPs, with significant impact on treatment planning. The added value of SPECT/CT over CI was 35.6%. SPECT/CT corrected CI classification and patient management in 27.9% of cases, while it down-staged the disease better than CI in 9.6% of cases. However, the combined use of CI and functional imaging, using
111In-Pentetreotide SPECT/CT, still leaves a big gap in the diagnostic pathway [
24]. Therefore, newer, higher-affinity SST analogs, labeled with radioisotopes with better resolution and dosimetry, such as
68Ga, which is a positron emitter, have been considered as promising SSTR imaging agents.
3. 68Ga-DOTA-Conjugated Peptide-Binding SSTR PET/CT
68Ga-DOTA-conjugated peptide-binding SSTR-based imaging techniques are acknowledged as a new category of radiopharmaceuticals that has recently been instructed into routine clinical practice, including DOTA-D Phe1-Tyr3-octreotide (DOTATOC), DOTA 1-Nal3-octreotide (DOTANOC), and DOTA-D Phe1-Tyr3-Thr8-octreotide (DOTATATE) [
25]. Although all of these radiopeptides bind to SSTR2, each has various affinities for other SSTR subtypes. In addition to the greatest affinity to SSTR2,
68Ga-DOTA-NOC also reveals a good affinity for SSTR3 and SSTR5;
68Ga-DOTA-TOC also shows a high affinity for SSTR5, but less affinity compared to DOTA-NOC, whereas
68Ga DOTA-TATE predominantly binds to SSTR2 [
26].
Table 1 lists the frequency of overexpression of different SSTR subtypes in different clinical presentations [
27].
Table 1. Expression of somatostatin receptors in different GEP-NENs (%).
| Tumor Types |
Receptor Subtypes |
| SSTR 1 |
SSTR 2 |
SSTR 3 |
SSTR 4 |
SSTR 5 |
| All GEP-NENs |
68 |
86 |
46 |
93 |
57 |
| Gastrinoma |
33 |
50 |
17 |
83 |
50 |
| Insulinoma |
33 |
100 |
33 |
100 |
67 |
| Glucagonoma |
67 |
100 |
67 |
67 |
67 |
| VIPoma |
100 |
100 |
100 |
100 |
100 |
| Mid-Gut NENs |
80 |
95 |
65 |
35 |
75 |
68Ga DOTA conjugated peptide-binding SSTR is generally used for primary tumor localization, including metastatic detection (staging) and evaluation of residual, recurrent, or progressive disease (restaging) [
28,
29] all the way through to the selection of patients for PRRT [
30]. PRRT can successfully control symptoms due to hypersecretion of hormones and has also been displayed to improve OS in progressive or symptomatic NETs patients [
31]. The mechanism of uptake is based on increased SSTR expression in neuroendocrine cells and can be detected on SSTR scanning [
32]. The previous studies revealed statistically significant decreased tumor uptake of
68Ga-DOTA-peptides PET in poorly differentiated NETs, and its uptake might be related to aggressive behavior and might lead to poorer prognosis [
33]. However, some cases in this study were low-grade NENs that showed low tumor uptake on
68Ga-DOTA-peptides PET/CT scans. It is possible that the FNA site may not fully reflect the true pathological grade of a patient with heterogeneity of cellular differentiation within the same tumor mass, and this may also reflect the potential ability of
68Ga-labeled SSTR PET/CT to map these cellular characteristics (
Figure 1) [
33,
34].
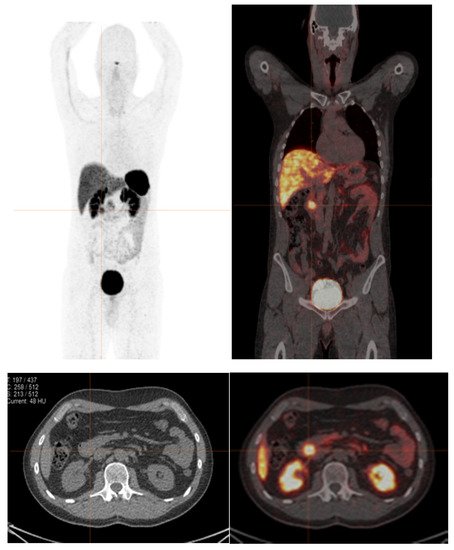
Figure 1. A 41-year-old male with recurrent NENs at the duodenum. 68Ga-DOTA-NOC PET/CT shows abnormal focal tracer uptake at the duodenum (SUVmax of 5.9), without other definite evidence of abnormal tracer uptake. Ki-67 index from endoscopic fine-needle aspiration of duodenum was 1%. It is possible that the FNA site may not fully reflect the true pathological grade of a patient with heterogeneity of cellular differentiation.
The recommended indications for patients with NENs according to EANM Guidelines for
68Ga-DOTA-conjugated somatostatin PET/CT imaging [
35] are as follows:
-
Localized primary tumors and detect sites of metastasis (staging);
-
Monitoring patients with known disease in the detection of residual, recurrent or progressive disease (re-staging);
-
Determine SSTR status (patients with SSTR positive tumors are more likely to respond to targeted therapy with SSA);
-
Select patients with metastasis for PRRT (177Lu or 90Y–DOTA-labelled peptides).
68Ga-DOTA-conjugated peptide-binding SSTR is significantly superior to
111In-Pentetreotide in both diagnostic accuracy and its therapeutic impact. Available evidence also supports the concept that
68Ga-DOTA-conjugated peptide-binding SSTR imaging often demonstrates tumor uptake in some patients with negative or equivocal
111In-Pentetreotide scans, and it identifies patients who may benefit from PRRT [
36].
A prospective study of 131 GEP-NENs patients determined the superiority of
68Ga-DOTATATE PET/CT over
111In-pentetreotide SPECT/CT, CT, and/or MRI.
68Ga-DOTATATE PET/CT detected 95.1% of lesions, while CI detected 45.3% of lesions, and
111In-pentetreotide SPECT/CT detected only 30.9% of lesions with statistically significant differences between all imaging modalities. In the subgroup of patients with carcinoid symptoms but negative biochemical testing,
68Ga-DOTATATE PET/CT detected lesions in 65.2% of patients. Of note, 40% of the lesions detected by
68Ga-DOTATATE PET/CT were missed on CI or
111In-pentetreotide SPECT/CT. Additionally, based on the findings from
68Ga-DOTATATE PET/CT, there was a significant change in the patient management in approximately a third of the patients (32.8%) [
37].
Binnebeek et al. revealed that significantly more lesions were detected on
68Ga-DOTATOC-PET/CT as compared to
111In-pentetreotide scans. The sensitivity for PET/CT was 99.9% (95% CI, 99.3–100.0), and for SPECT, it was 60.0% (95% CI, 48.5–70.2). The organ-by-organ analysis showed that the PET was most frequently visualized in the liver and skeleton. They concluded that
68Ga-DOTATOC-PET/CT is superior for detecting NENs compared to
111In-pentetreotide SPECT [
38]. Additionally, treatment was changed in more than one-third of patients with SSTR PET/CT rather than
111In-pentetreotide scan [
39].
4. 18F-FDG PET/CT
18F-FDG is used to evaluate glucose metabolism, and it is the most commonly used radiotracer in PET imaging. It is not currently routinely used for NENs imaging, but recent experience has suggested that it can provide complementary information.
18F-FDG PET/CT has a limited advantage in well-differentiated NETs, since most NENs frequently have near normal glucose metabolism [
40].
18F-FDG exploits the increased glycolytic activity of tumor cells, as glycolytic activity seems to be low in most NENs. In fact, it can differentiate between slowly proliferative and aggressive tumors [
41,
42]. Additionally,
18F-FDG PET/CT scans can allow prognostic prediction, including overall survival (OS) and progression free survival (PFS). Finally, it could potentially identify those who are not responding earlier, characterize early progression, and change therapy if required.
Assessment of glucose metabolism by
18F-FDG may be helpful in the diagnosis of high-risk aggressive disease associated with poor outcomes.
18F-FDG PET may still an essential modality in the management of NENs patients, due to its better prognostic value and greater sensitivity to identify the extent of disease, particularly in aggressive high-grade tumors [
43] Bahri H et al. revealed that the median PFS and OS are significantly greater in patients with a negative
18F-FDG PET finding (OS nearly 120 months) than in patients with a positive
18F-FDG PET finding (only 15 months) [
44]. Ezziddin et al. [
45] retrospectively reviewed data from 89 patients with metastatic GEP-NENs and classified three different prognostic groups according to FDG uptake: mG1, tumor-to-liver ratio of maximum SUV ≤ 1.0; mG2, 1.0–2.3; and mG3, >2.3. These groups were correlated with significantly different OS (not more than 114 vs. 55 vs. 13 months, respectively). As mentioned,
18F-FDG PET must not only be used in staging but also for grading whole-body imaging modality; positive
18F-FDG PET lesions have a significant correlation with prognosis, irrespective of SSTR expression.
The recommended indications for
18F-FDG PET/CT in patients with NENs according to EANM Guideline for
68Ga-DOTA-conjugated somatostatin PET/CT imaging [
35] are as follows: