The soma, dendrites and axon of neurons may display calcium-dependent release of transmitters and peptides. Such release is named extrasynaptic for occurring in absence of synaptic structures. Emphasis is given to the somatic release of serotonin by the classical leech Retzius neuron, which has allowed detailed studies on the fine steps from excitation to exocytosis. Trains of action potentials induce transmembrane calcium entry through L-type channels. For action potential frequencies above 5 Hz, summation of calcium transients on individual action potentials activates the second calcium source: ryanodine receptors produce calcium-induced calcium release. The resulting calcium tsunami activates mitochondrial ATP synthesis to fuel transport of vesicles to the plasma membrane. Serotonin that is released maintains a large-scale exocytosis by activating the third calcium source: serotonin autoreceptors coupled to phospholipase C promote IP3 production. Activated IP3 receptors in peripheral endoplasmic reticulum release calcium that promotes vesicle fusion. The Swiss-clock workings of the machinery for somatic exocytosis has a striking disadvantage. The essential calcium-releasing endoplasmic reticulum near the plasma membrane hinders the vesicle transport, drastically reducing the thermodynamic efficiency of the ATP expenses and elevating the energy cost of release.
- calcium channels
- somatic exocytosis
- serotonin
- extrasynaptic release
- thermodynamic efficiency
- leech
1. Introduction
2. Electrical Activity and Calcium Promote Vesicle Transport and Somatic Exocytosis
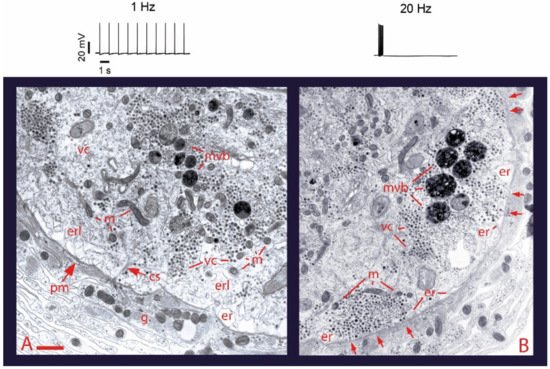
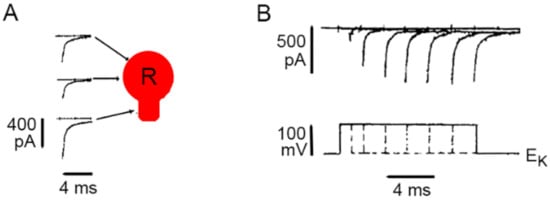
3. Calcium Enters the Neuronal Soma through L-Type Channels
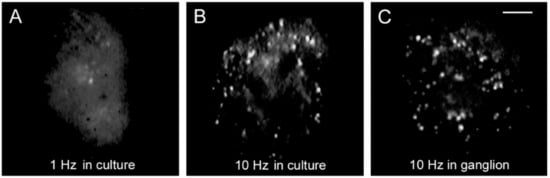
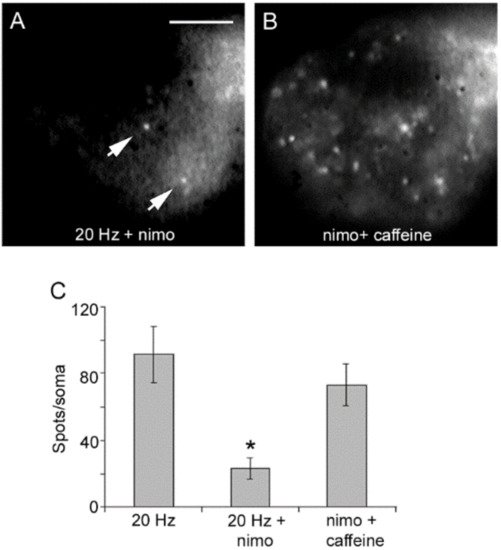
4. High-Frequency Activation of L-Type Channels Promotes Somatic Exocytosis
References
- Huang, L.Y.; Neher, E. Ca(2+)-dependent exocytosis in the somata of dorsal root ganglion neurons. Neuron 1996, 17, 135–145.
- Jaffe, E.H.; Marty, A.; Schulte, A.; Chow, R.H. Extrasynaptic vesicular transmitter release from the somata of substantia nigra neurons in rat midbrain slices. J. Neurosci. 1998, 18, 3548–3553.
- Puopolo, M.; Hochstetler, S.E.; Gustincich, S.; Wightman, R.M.; Raviola, E. Extrasynaptic release of dopamine in a retinal neuron: Activity dependence and transmitter modulation. Neuron 2001, 30, 211–225.
- Kaushalya, S.K.; Desai, R.; Arumugam, S.; Ghosh, H.; Balaji, J.; Maiti, S. Three-photon microscopy shows that somatic release can be a quantitatively significant component of serotonergic neurotransmission in the mammalian brain. J. Neurosci. Res. 2008, 86, 3469–8340.
- Huang, H.P.; Zhu, F.P.; Chen, X.W.; Xu, Z.Q.; Zhang, C.X.; Zhou, Z. Physiology of quantal norepinephrine release from somatodendritic sites of neurons in locus coeruleus. Front. Mol. Neurosci. 2012, 5, 29.
- Trueta, C.; De-Miguel, F.F. Extrasynaptic exocytosis and its mechanisms: A source of molecules mediating volume transmission in the nervous system. Front. Physiol. 2012, 3, 319.
- Sulzer, D.; Cragg, S.J.; Rice, M.E. Striatal dopamine neurotransmission: Regulation of release and uptake. Basal Ganglia 2016, 6, 123–148.
- Grinevich, V.; Ludwig, M. The multiple faces of the oxytocin and vasopressin systems in the brain. J. Neuroendocr. 2021, 33, e13004.
- Borroto-Escuela, D.O.; Perez De La Mora, M.; Manger, P.; Narváez, M.; Beggiato, S.; Crespo-Ramírez, M.; Navarro, G.; Wydra, K.; Díaz-Cabiale, Z.; Rivera, A.; et al. Brain Dopamine Transmission in Health and Parkinson’s Disease: Modulation of Synaptic Transmission and Plasticity Through Volume Transmission and Dopamine Heteroreceptors. Front. Syn. Neurosci. 2018, 10, 20.
- Newman, E.A. Glial cell regulation of neuronal activity and blood flow in the retina by release of gliotransmitters. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2015, 370, 20140195.
- Igelhorst, B.A.; Niederkinkhaus, V.; Karus, C.; Lange, M.D.; Dietzel, I.D. Regulation of neuronal excitability by release of proteins from glial cells. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2015, 370, 20140194.
- Kravitz, E.A. Hormonal control of behavior: Amines and the biasing of behavioral output in lobsters. Science 1988, 241, 1775–1781.
- Hirasawa, H.; Contini, M.; Raviola, E. Extrasynaptic release of GABA and dopamine by retinal dopaminergic neurons. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370, 20140186.
- Ludwig, M.; Apps, D.; Menzies, J.; Patel, J.C.; Rice, M.E. Dendritic Release of Neurotransmitters. Comp. Physiol. 2016, 7, 235–252.
- Quentin, E.; Belmer, A.; Maroteaux, L. Somato-Dendritic Regulation of Raphe Serotonin Neurons; A Key to Antidepressant Action. Front. Neurosci. 2018, 12, 982.
- Hökfelt, T.; Barde, S.; Xu, Z.D.; Kuteeva, E.; Rüegg, J.; Le Maitre, E.; Risling, M.; Kehr, J.; Ihnatko, R.; Theodorsson, E.; et al. Neuropeptide and Small Transmitter Coexistence: Fundamental Studies and Relevance to Mental Illness. Front. Neural. Circ. 2018, 12, 106.
- Delgado-Lezama, R.; Bravo-Hernández, M.; Franco-Enzástiga, Ú.; De la Luz-Cuellar, Y.E.; Alvarado-Cervantes, N.S.; Raya-Tafolla, G.; Martínez-Zaldivar, L.A.; Vargas-Parada, A.; Rodríguez-Palma, E.J.; Vidal-Cantú, G.C.; et al. The role of spinal cord extrasynaptic α5 GABAA receptors in chronic pain. Physl. Rep. 2021, 16, e14984.
- Wacker, D.; Ludwig, M. The role of vasopressin in olfactory and visual processing. Cell Tis. Res. 2021, 375, 201–215.
- Brown, C.H.; Ludwig, M.; Tasker, J.G.; Stern, J.E. Somato-dendritic vasopressin and oxytocin secretion in endocrine and autonomic regulation. J. Neuroendocr. 2020, 32, e12856.
- Livero, G. Somatostatin, a Presynaptic Modulator of Glutamatergic Signal in the Central Nervous System. Int. J. Mol. Sci. 2021, 22, 5864.
- Tobin, V.A.; Ludwig, M. The role of the actin cytoskeleton in oxytocin and vasopressin release from rat supraoptic nucleus neurons. J. Physiol. 2007, 582, 1337–1348.
- De-Miguel, F.F.; Leon-Pinzon, C.; Torres-Platas, S.G.; Del-Pozo, V.; Hernández-Mendoza, G.A.; Aguirre-Olivas, D.; Mendez, B.; Moore, S.; Sánchez-Sugía, C.; García-Aguilera, M.A.; et al. Extrasynaptic Communication. Front. Mol. Neurosci. 2021, 14, 638858.
- De-Miguel, F.F.; Leon-Pinzon, C.; Noguez, P.; Mendez, B. Serotonin release from the neuronal cell body and its long-lasting effects on the nervous system. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370, 20140196.
- Mesce, K.A.; Esch, T.; Kristan, W.B., Jr. Cellular substrates of action selection: A cluster of higher-order descending neurons shapes body posture and locomotion. J. Comp. Physiol. A 2008, 194, 469–481.
- Ashaber, M.; Tomina, Y.; Kassraian, P.; Bushong, E.A.; Kristan, W.B.; Ellisman, M.H.; Wagenaar, D.A. Anatomy and activity patterns in a multifunctional motor neuron and its surrounding circuits. eLife 2021, 10, e61881.
- De-Miguel, F.F.; Santamaría-Holek, I.; Noguez, P.; Bustos, C.; Hernández-Lemus, E.; Rubí, J.M. Biophysics of active vesicle transport, an intermediate step that couples excitation and exocytosis of serotonin in the neuronal soma. PLoS ONE 2012, 460, e45454.
- Ma, P.M.; Beltz, B.S.; Kravitz, E.A. Serotonin-containing neurons in lobsters: Their role as gain-setters in postural control mechanisms. J. Neurophysiol. 1992, 68, 36–54.
- García-Pérez, E.; Vargas-Caballero, M.; Velazquez-Ulloa, N.; Minzoni, A.; De-Miguel, F.F. Synaptic integration in electrically coupled neurons. Biophys. J. 2004, 86, 646–655.
- Aghajanian, G.K.; Foote, W.E.; Sheard, M.H. Lysergic acid diethylamide: Sensitive neuronal units in the midbrain raphe. Science 1968, 161, 706–708.
- Mosko, S.S.; Jacobs, B.L. Midbrain raphe neurons: Spontaneous activity and response to light. Phys. Behav. 1974, 13, 589–593.
- Trueta, C.; Kuffler, D.P.; De-Miguel, F.F. Cycling of dense core vesicles involved in somatic exocytosis of serotonin by leech neurons. Front. Physiol. 2012, 3, 175.
- Trueta, C.; Méndez, B.; De-Miguel, F.F. Somatic exocytosis of serotonin mediated by L-type calcium channels in cultured leech neurones. J. Physiol. 2003, 547, 405–416.
- Trueta, C.; Sánchez-Armass, S.; Morales, M.A.; De-Miguel, F.F. Calcium-induced calcium release contributes to somatic secretion of serotonin in leech Retzius neurons. J. Neurobiol. 2004, 61, 309–316.
- Fu, X.W.; Nurse, C.A.; Wong, V.; Cutz, E. Hypoxia-induced secretion of serotonin from intact pulmonary neuroepithelial bodies in neonatal rabbit. J. Physiol. 2002, 539, 503–510.
- Simmons, M.L.; Terman, G.W.; Gibbs, S.M.; Chavkin, C. L-type calcium channels mediate dynorphin neuropeptide release from dendrites but not axons of hippocampal granule cells. Neuron 1995, 14, 1265–1272.
- Shibuya, I.; Noguchi, J.; Tanaka, K.; Harayama, N.; Inoue, U.; Kabashima, N.; Ueta, Y.; Hattori, Y.; Yamashita, H. PACAP increases the cytosolic Ca2+ concentration and stimulates somatodendritic vasopressin release in rat supraoptic neurons. J. Neuroendocr. 1998, 10, 31–42.
- De Kock, C.P.; Burnashev, N.; Lodder, J.C.; Mansvelder, H.D.; Brussaard, A.B. NMDA receptors induce somatodendritic secretion in hypothalamic neurones of lactating female rats. J. Physiol. 2004, 561, 53–64.
- De Kock, C.P.; Cornelisse, L.N.; Burnashev, N.; Lodder, J.C.; Timmerman, A.J.; Couey, J.J.; Mansvelder, H.D.; Brussaard, A.B. NMDA receptors trigger neurosecretion of 5-HT within dorsal raphe nucleus of the rat in the absence of action potential firing. J. Physiol. 2006, 577, 891–905.
- Fernandez de Miguel, F.; Cooper, R.; Adams, W.B. Synaptogenesis and calcium current distribution in cultured leech neurons. Proc. R. Soc. Lond. B 1992, 247, 215–221.
- Leon-Pinzon, C.; Cercós, M.G.; Noguez, P.; Trueta, C.; De-Miguel, F.F. Exocytosis of serotonin from the neuronal soma is sustained by a serotonin and calcium-dependent feedback loop. Front. Cell Neurosci. 2014, 8, 169.
- Dreifuss, J.J.; Kalnins, I.; Kelly, J.S.; Ruf, K.B. Action potentials and release of neurohypophysial hormones in vitro. J. Physiol. 1971, 215, 805–817.
- Hörner, M.; Weiger, W.A.; Edwards, D.H.; Kravitz, E.A. Excitation of identified serotonergic neurons by escape command neurons in lobsters. J. Exp. Biol. 1997, 200, 2017–2033.
- Kirischuk, S.; Voitenko, N.; Kostyuk, P.; Verkhratsky, A. Calcium signalling in granule neurones studied in cerebellar slices. Cell Calcium 1996, 19, 59–71.
- Shmigol, A.; Svichar, N.; Kostyuk, P.; Verkhratsky, A. Gradual caffeine-induced Ca2+ release in mouse dorsal root ganglion neurons is controlled by cytoplasmic and luminal Ca2+. Neuroscience 1996, 73, 1061–1067.
- Thayer, S.A.; Hirning, L.D.; Miller, R.J. The role of caffeine-sensitive calcium stores in the regulation of the intracellular free calcium concentration in rat sympathetic neurons in vitro. Mol. Pharm. 1988, 34, 664–673.
- Usachev, Y.; Shmigol, A.; Pronchuk, N.; Kostyuk, P.; Verkhratsky, A. Caffeine-induced calcium release from internal stores in cultured rat sensory neurons. Neuroscience 1993, 57, 845–859.
