White meat is the nutritional term for lighter-colored meat that contains less myoglobin than red meat, which contains a great deal. White meat includes poultry (e.g., chicken, duck, goose and turkey), fish, reptiles (e.g., land snail), amphibians (e.g., frog), crustaceans (e.g., shrimp and crab) and bivalves (e.g., oyster and clam), but it excludes all mammal flesh such as beef, pork, and lamb. White meat has high nutritional value and plays an important role in human diet. The production and sale of white meat need to meet specific quality and safety standards. Fluorescence spectroscopy, color imaging and multispectral imaging (MSI) have emerged as effective analytical methods for the non-destructive detection of quality attributes of various white meat products such as fish, shrimp, chicken, duck and goose.
1. Introduction
As a global issue, food safety and quality are of increasing concern to companies and customers
[1]. White meat is the nutritional term for lighter-colored meat that contains less myoglobin than red meat, which contains a great deal. Compared with white meat, the intake of red meat has a greater correlation with colorectal cancer (CRC), indicating that white meat intake is more beneficial to human health
[2]. White meat includes poultry (e.g., chicken, duck, goose and turkey), fish, reptiles (e.g., land snail), amphibians (e.g., frog), crustaceans (e.g., shrimp and crab) and bivalves (e.g., oyster and clam), but it excludes all mammal flesh such as beef, pork, and lamb. White meat has high nutritional value and plays an important role in human diet. The production and sale of white meat need to meet specific quality and safety standards. The freshness of fish is one of the important indicators for evaluating its quality because of its high perishability
[3]. Moreover, poultry products are particularly susceptible to oxidation as this meat contains relatively high levels of unsaturated fatty acids and low levels of natural antioxidants, such as vitamin E. In addition, chemical residues in white meat may have an adverse effect on human health. For example, fluoroquinolone antibiotics are effective against a wide range of Gram-negative and positive bacteria, thus they are widely used in the medical and veterinary fields. However, their use in animals has raised concerns, as this practice may lead to an increase in microbial resistance
[4]. Moreover, nitrofuran drugs (NFs), including furazolidone (FZD), nitrofurazone (NFZ), and furantazone (FTD) are broad-spectrum antimicrobials. The potential risk of these compounds to human health is of great concern because of their carcinogenic and mutagenic properties. It is therefore crucial to ensure the quality and safety of white meat.
Traditional methods for meat quality and safety evaluation, such as manual inspection, mechanical and chemical methods, are time-consuming and destructive, and cannot meet the requirements of rapid inspection
[5]. For example, methods for freshness evaluation are based on human sensory qualities, such as appearance, taste and texture. However, human senses exhibit a very high degree of subjectivity and can therefore be questioned in certain situations
[3]. Even if manual inspection could meet accuracy requirements, it is still a labor-intensive and time-consuming process. Recently, the meat industry has adopted the most advanced high-speed processing technologies, and meat processors need fast, non-destructive, easy-to-use techniques to control the safety and quality of meat and meat products in order to achieve economic benefits. The requirement for real-time monitoring of food has encouraged the development of non-destructive measurement systems
[6]. Optical technology is becoming increasingly important in research and industrial applications to measure the quality attributes of meat and meat products in real time, non-destructively and accurately
[7]. Among these, the use of neural network-based RGB imaging technology has become very popular in recent years
[8]. In addition, fluorescence spectroscopy and multispectral imaging (MSI) also show obvious advantages and capabilities in the non-destructive evaluation of white meat.
There have been several reviews of these new techniques of meat quality assessment. These papers show that these spectroscopic methods have been implemented as an alternative to traditional methods, but they mainly focus on one technique for quality detection of one specific category of meat, e.g., fish
[3], shrimp
[4], chicken
[9], duck
[10], or red meat
[11]. As far as
thwe
researchers know, there is no literature review analyzing the application of various imaging techniques in the non-destructive quality inspection of various white meats. (The published reviews based on these three imaging techniques are tabulated in
Table 1).
Table 1. Summary of reviews on fluorescence spectroscopy, RGB- and MSI techniques in food evaluation.
|
Technology
|
Product
|
Target Attributes
|
Reference
|
|
MSI
|
Meat
|
Adulteration
|
Ropodi et al. [12]
|
|
MSI, HSI
|
Meat
|
Defects
|
Feng et al. [13]
|
|
MSI
|
Fish
Food
|
Quality
|
Su and Sun |
]. Data obtained from pure RGB imaging has been shown to be inferior to data obtained through spectral imaging when analyzing the quality of ground meat.
Figure 2.
Diagram of the RGB vision system used to obtain color images of pure and contaminated meat samples [33].
A multispectral image is a collection of grey-scale images. Each corresponds to a specific wavelength or band of wavelengths in the electromagnetic spectrum
[36]. MSI is a method of capturing images from different spectral bands with the aim of obtaining spatial and spectral information. Imagers based on MSI technology can provide wavelength channels in the near-UV, visible, near-IR, mid-IR and far-IR
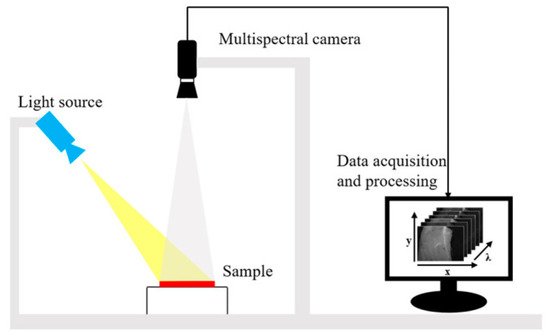
[37]. Thus, MSI can provide more information than RGB images. The acquired wavelength channels can be used directly for real-time applications in certain fields (e.g., fruit packing plants and food processing plants). A typical MSI system is shown in
Figure 3. The system uses an adjustable focus lens to achieve high resolution imaging of 1290 × 960 pixels and has six bands, each covering a relatively wide range of wavelengths, which is strong for fast imaging
[38].
Figure 3. The MSI system consists of a light source (HL-2000-FHSA; Ocean Optics, Dunedin, FL, USA) and focusable lens (Nikon, Tokyo, Japan) plus a multi-channel spectral camera (miniCAM5; QHY-CCD, China)
[38].
3. Quality Evaluation of White Meat
The application of fluorescence spectroscopy, RGB imaging and MSI for white meat quality inspection has been thoroughly and extensively researched as shown in Table 2. For MSI techniques, correlation coefficient (R) or coefficient of determination (R2) is an important statistical metric for assessing model fit, while root mean square error (RMSE) is considered an indicator of the sample standard deviation between measured and actual values, indicating that a well-performing model should obtain a high R or R2 value and a low RMSE value. There are many different judgements due to the variability and multiplicity of the techniques.
Table 2. Applications of fluorescence spectroscopy, RGB imaging and MSI for quality evaluation of various white meat products.
|
White Meat
|
Module
|
Quality Parameters
|
Accuracy
|
Reference
|
|
Fish
|
MSI
|
TVB-N,
PPC
|
R2p = 0.862 for TVB-N,
R2p = 0.921 for PPC
|
Khoshnoudi-Nia and Moosavi-Nasab [39], Khoshnoudi-Nia and Moosavi-Nasab [40]
|
|
Fish
|
MSI
|
TVC
|
R2 = 0.62
|
Govari, et al. [41 |
|
| MSI
| [ | 14]
|
] |
|
TVC
|
R2 = 0.683
|
Fengou, et al. [42]
|
MSI, IRS, SERS, LIBS and HSI
|
Food
|
Quality
|
Wang et al. [15]
|
|
Fish
|
MSI
|
Astaxanthin concentration
|
R2 = 0.86
|
Dissing, et al. [43]
|
MSI, HSI and VS
|
|
Fish
|
Food
|
|
MSI
Authenticity, quality and safety
|
TVB-N,
TBARS,
Ropodi et al. [16]
|
|
| K |
|
R2p = 0.922 for TVB-N,
R2p = 0.867 for TBARS,
R2p = 0.936 for K
|
Cheng, et al. [44]
|
Fluorescence spectroscopy
|
Food
|
Quality
|
Karoui and Blecker [17]
|
|
Fish
|
MSI
|
A ‘standard freshness index’ of K
|
R2 = 0.94,
|
Omwange, et al. [45]
|
Fluorescence spectroscopy
|
|
Fish
|
Food
|
Fluorescence spectroscopy
Quality
|
A ‘standard freshness index’ of K
Strasburg and Ludescher [18]
|
|
| R | 2 | = 0.92
|
Omwange, et al. [46]
|
Visible/Infrared, Raman and Fluorescence spectroscopy
|
|
|
Fish
| Raw and processed food
|
Fluorescence spectroscopy
Quality
|
|
A ‘standard freshness index’ of K
He and Sun [19]
|
|
| R | 2 | = 0.95
|
Liao, et al. [47]
|
Fluorescence spectroscopy
|
Food
|
|
Fish
|
Fluorescence spectroscopy
|
Quality
|
AEC;
NADH
Ahmad et al. [20]
|
|
| R | 2 | = 0.90 for AEC,
R2 = 0.85 for NADH
|
Fluorescence spectroscopy
|
Dairy products
|
Quality and safety
|
Shaikh and O’Donnell [21]
|
|
|
|
Muscle classification
|
|
|
| Rahman, et al. | [ | 48]
|
|
Fish
|
Fluorescence spectroscopy
|
NADH
|
90.5%
|
Hassoun and Karoui [49]
|
Fluorescence spectroscopy
|
Fresh and frozen-thawed muscle foods |
Hassoun |
|
Fish
|
RGB imaging
| [ |
Classification performance
22]
|
|
| 99.5% |
|
| Park, et al. [50]
|
RGB-Imaging
|
Meat
|
|
Fish
|
| Quality and safety
|
Taheri-Garavand et al. [ |
RGB imaging23]
|
|
|
| Astaxanthin concentration
|
R2 = 0.66
|
Dissing et al. [43]
|
RGB-Imaging
|
Fish
|
Quality
|
Dowlati et al. [24]
|
|
RGB-Imaging
|
Food
|
Quality
|
Gomes and Leta [25]
|
|
RGB-Imaging
|
Food
|
Quality
|
Amani et al. [26]
|
MSI––Multispectral imaging; HSI––Hyperspectral imaging; IRS––Infrared spectroscopy; SERS––Surface-Enhanced Raman Spectroscopy; LIBS––Laser induced breakdown spectroscopy; VS––Vibrational Spectroscopy.
2. Fluorescence Spectroscopy, RGB- and Multispectral-Imaging
Fluorescence spectroscopy has proven to be an effective analytical technique over the last decade for monitoring the properties of various food products
[27]. The number of published papers and citations on the use of fluorescence spectroscopy to study food quality and/or authenticity has increased exponentially over the last decade. Fluorescence is the emission of light by a fluorophore following the absorption of ultraviolet or visible light
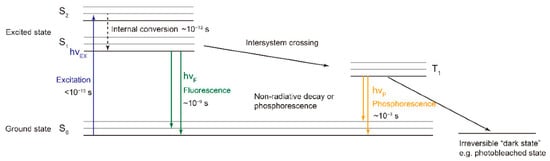
[28]. Fluorophores absorb energy as light at specific wavelengths and release energy as light at higher wavelengths. The Jablonski diagram in
Figure 1 illustrates the electron energy levels of fluorophores, with the jumps between them indicated by arrows
[29]. Fluorescent compounds are highly sensitive to their environment, so fluorescence can be used to characterize the conformational changes that occur under different production and storage conditions
[21]. For specific applications, fluorescence analysis has the lowest background levels, low detection limits and is readily available in most laboratories
[30].
Figure 1.
Jablonski diagram of the electron energy levels and transitions of fluorophores [29].
RGB imaging or color imaging has gained popularity due to its clear color rendering principle, simple hardware structure and mature production process. RGB images are captured by digital cameras, webcams, or scanners from computer vision systems. These systems, typically containing an illumination system, camera and image analysis software using a computer
[31], are capable of retrieving color information from captured images in the form of pixel ribbons of RGB
[32].
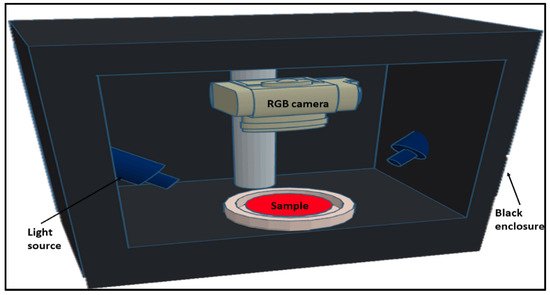
Figure 2, for example, shows an RGB vision system for capturing color images of pure and adulterated meat samples
[33]. RGB imaging has been shown to determine the general color and visual appearance of samples
[34]. This imaging technology is valuable in the meat industry because it is simple, low cost and non-destructive. However, even though RGB imaging has many advantages, it only provides spatial information at a limited number of wavelengths. Conventional RGB imaging systems can be poor at identifying sensitive surface features in wavelengths other than RGB
[35
|
|
Fish |
|
|
|
|
RGB imaging |
|
|
|
|
Freshness of tuna meat cuts |
|
|
|
| 86.67% |
|
| Lugatiman, et al. [51]
|
|
Fish
|
RGB imaging
|
The main color of the sample
|
75%
|
Mateo, et al. [52]
|
|
Fish
|
RGB imaging
|
Texture features
|
86.3%
|
Gu, et al. [53]
|
|
Fish
|
RGB imaging
|
Color of Salmon Fillets
|
R = 0.95
|
Quevedo, et al. [54]
|
|
Fish
|
RGB imaging
|
Gill and eye color changes in the sparus aurata
|
R2 = 0.994
|
Dowlati, et al. [55]
|
|
Fish
|
RGB imaging
|
Body color of carp
|
94.97%
|
Taheri-Garavand, et al. [56]
|
|
Fish
|
RGB imaging
|
Freshness
|
98.2%
|
Rocculi, et al. [57]
|
|
Shrimp
|
Fluorescence spectroscopy
|
4-hexylresorcinol
|
81.6%
|
Jonker and Dekker [58]
|
|
Shrimp
|
Fluorescence spectroscopy
|
K, pH
|
R2 = 0.80
|
Rahman, et al. [59]
|
|
Shrimp
|
RGB imaging
|
pH
|
100%
|
Witjaksono, et al. [60]
|
|
Shrimp
|
RGB imaging
|
Identification accuracy of the proposed ShrimpNet for shrimp
|
95.48%
|
Hu, et al. [61]
|
|
Shrimp
|
RGB imaging
|
Shrimp dehydration levels
|
R = 0.86
|
Mohebbi, et al. [62]
|
|
Shrimp
|
RGB imaging
|
Color changes in the head, legs and tail of pacific white shrimp (litopenaeus vannamei)
|
90%
|
Ghasemi-Varnamkhasti, et al. [63]
|
|
Chicken
|
Fluorescence spectroscopy
|
Hydroxyproline concentration
|
R2 = 0.82
|
Monago-Maraña, et al. [64]
|
|
Chicken
|
MSI
|
Skin tumors
|
86%
|
Chao, et al. [65]
|
|
Chicken
|
MSI
|
TVC
|
90.4%
|
Spyrelli, et al. [66]
|
|
Chicken
|
MSI
|
pork-chicken adulteration
|
90.00% for fresh samples, 86.67% for frozen-thawed samples
|
Fengou, et al. [67]
|
|
Chicken
|
MSI
|
Sepsis in chickens
|
98.6% for septic chickens,
96.3% for healthy chickens
|
Yang, et al. [68]
|
|
Chicken
|
MSI
|
Contamination detection
|
96%
|
Park, et al. [69]
|
|
Chicken
|
MSI
|
Chicken heart disease characterization
|
100%
|
Chao, et al. [70]
|
|
Chicken
|
MSI;
Fluorescence spectroscopy
|
Contamination detection
|
92.5%
|
Seo, et al. [71]
|
|
Chicken
|
Fluorescence spectroscopy
|
Lipid oxidation
|
R = 0.73
|
Gatellier, et al. [72]
|
|
Chicken
|
Fluorescence spectroscopy
|
P. aeruginosa concentration
|
96%
|
Abdel-Salam, et al. [73]
|
|
Chicken
|
Fluorescence spectroscopy
|
chicken meat tenderness
|
R = 0.870
|
Yu, et al. [74]
|
|
Chicken
|
Fluorescence spectroscopy
|
Contamination detection
|
96.6%
|
Cho, et al. [75]
|
|
Chicken
|
Fluorescence spectroscopy
|
Measurement of lipid oxidation
|
98%
|
Wold and Kvaal [76]
|
|
Chicken
|
RGB imaging
|
Avian flu infected chickens
|
97.43%
|
Cuan, et al. [77]
|
|
Chicken
|
RGB im-aging
|
Color
|
94%
|
Yumono, et al. [78]
|
|
Chicken
|
RGB im-aging
|
Freshness
|
R = 0.987
|
Taheri-Garavand, et al. [79]
|
|
Duck
|
Fluorescence spectroscopy
|
Gentamicin Residual in Duck Meat
|
R = 0.996
|
Wang, et al. [80]
|
|
Duck
|
Fluorescence spectroscopy
|
Doxycycline content in duck meat
|
R = 0.998
|
Wang, et al. [81]
|
|
Duck
|
Fluorescence spectroscopy
|
Carbaryl residue in duck meat
|
R = 0.976
|
Xiao et al. [10]
|
|
Duck
|
Fluorescence spectroscopy
|
Tetracycline content
|
R = 0.952
|
Zhao, et al. [82]
|
|
Duck
|
Fluorescence spectroscopy
|
Triazophos content
|
R2p = 0.974,
|
Zhao, et al. [83]
|
|
Duck
|
Fluorescence spectroscopy
|
Neomycin residue
|
R = 0.999
|
Jiang, et al. [84]
|
|
Duck
|
Fluorescence spectroscopy
|
Carbofuran residue
|
R2p = 0.999
|
XIAO, et al. [85]
|
TVB-N––total volatile basic nitrogen; PPC—Psycho-trophic Plate Count; TVC—total viable count; LDA—Linear Discriminant Analysis; MD—Mahalanobis distance; PCA—Principal component analysis; m—mean; TBARS—Thio-barbituric acid reactive substances; AEC—adenylate energy charge; NAD and NADH—nicotinamide adenine dinucleotide; CFU—colony-forming units; TBARS—thio-barbituric acid reactive substances.