Calcineurin, a calcium-dependent serine/threonine phosphatase, integrates the alterations in intracellular calcium levels into downstream signaling pathways by regulating the phosphorylation states of several targets. Intracellular Ca2+ is essential for normal cellular physiology and cell cycle progression at certain critical stages of the cell cycle. Recently, it was reported that calcineurin is activated in a variety of cancers. Given that abnormalities in calcineurin signaling can lead to malignant growth and cancer, the calcineurin signaling pathway could be a potential target for cancer treatment. For example, NFAT, a typical substrate of calcineurin, activates the genes that promote cell proliferation. Furthermore, cyclin D1 and estrogen receptors are dephosphorylated and stabilized by calcineurin, leading to cell proliferation.
- cancer
- cell cycle
- intracellular calcium ions
- calcineurin
- dephosphorylation
1. Introduction
2. Cell Cycle and CDK
Cell division is precisely regulated by a series of CDKs, whose activity is dependent on the binding to different cell cycle-specific cyclins [15][14]. Cyclins confer substrate specificity and form CDK/cyclin complexes, which activate or inactivate target proteins by phosphorylation, and orchestrate coordinated cell cycle progression. In addition to CDK association with cyclins, the activity of CDKs is tightly regulated by several mechanisms, including phosphorylation, binding of CDK inhibitors (CKIs), and subcellular localization of CDK/cyclin complexes [16][15]. One of the critical targets of the CDK-cyclin complex in the G1 phase is the retinoblastoma protein (Rb). In the early G1 phase, CDK4/6-cyclin D initiates the phosphorylation of Rb, and, subsequently, CDK2-cyclin E phosphorylates Rb [17,18,19,20][16][17][18][19]. Phosphorylation of Rb releases the E2F transcription factor, and promotes the expression of the genes required for cell cycle progression [21,22][20][21]. This enables cells to pass through the restriction point at the G1/S boundary and to commence the S phase. CDK2-cyclin A plays an important role in S phase progression through the phosphorylation of proteins involved in DNA replication [23,24][22][23]. As cells enter the S phase, the CDK2-cyclin A complex is activated and remains activated throughout the G2 phase [25,26][24][25]. In the late G2 phase, CDK1-cyclin B is activated, allowing for the entry of cells into mitosis [27][26]. During G2/M transition, CDK1-cyclin A activity is necessary for prophase initiation [28][27].3. Calcium, Calmodulin, and Calmodulin-Dependent Protein Kinases in Cell Proliferation
In mammalian cells, Ca2+ is required at several different points during the cell cycle. Cells are most sensitive to the depletion of extracellular Ca2+ at early G1 and near the G1/S boundary [12,29][11][28]. In early G1, Ca2+ regulates the expression of immediate-early genes, such as FOS, JUN, and MYC. Ca2+ is also required for Rb phosphorylation at the G1/S boundary. Spontaneous Ca2+ oscillations at the G1/S phase transition have been described in synchronized immortalized cell lines. These Ca2+ oscillations are accompanied by DNA replication [30][29]. Once hormone receptors are activated, intracellular Ca2+ levels increase. Ca2+ regulates certain targets directly and others indirectly through Ca2+-binding proteins, such as CaM. Ca2+/CaM activates various pathways involved in the regulation of cellular processes, such as secretion, cell motility, ion homeostasis, and gene transcription. CaM also modulates a large number of intracellular enzymes, including protein kinases, protein phosphatases, phosphodiesterases, adenylyl cyclases, and ion channels [31][30]. CaM is required for cell cycle progression through G1, specifically the G1/S transition. Consistent with this notion, CaM antagonists block cell cycle progression in early or late G1 phases [32][31]. Furthermore, the exit from mitosis is sensitive to changes in the concentration of CaM [33][32]. Upon binding with Ca2+, CaM undergoes a major conformational change, and forms a Ca2+/CaM complex to interact with a variety of target proteins such as cellular kinases [34][33]. The best-studied kinases involved in Ca2+/CaM signaling are the CaMKs [35,36][34][35]. CaN and CaMK play important roles in cell cycle progression by activating or inhibiting key cell cycle regulators (Figure 1). The CaN/NFAT pathway contributes to inducing the transcription of cyclin D1 [37][36] and CDK4, and stabilizes cyclin D1 by dephosphorylating Thr286 of cyclin D [38][37].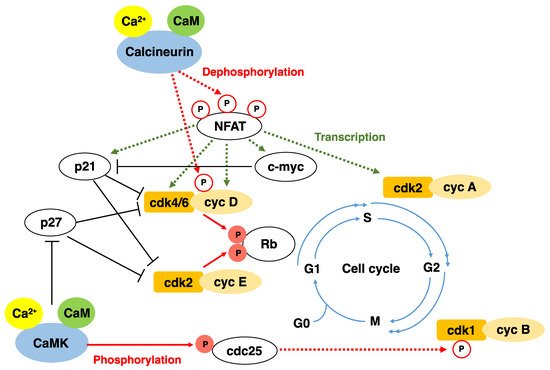
4. Characteristics of Calcineurin
CaN, also known as protein phosphatase 2 B (PP2B), is a serine/threonine protein phosphatase that is conserved in all eukaryotes [54,55,56,57][38][39][40][41]. CaN is a heterodimer composed of a catalytic subunit, calcineurin A (CnA), and a Ca2+-binding regulatory subunit, calcineurin B (CnB). In mammals, three independent genes, PPP3CA, PPP3CB, and PPP3CC, encode CnAα, CnAβ, and CnAγ, respectively. CnAα and CnAβ exhibit ubiquitous expression patterns, whereas the CnAγ expression is restricted to the testis and brain [58,59,60,61][42][43][44][45]. The CaN regulatory subunits CnB1 and CnB2 are encoded by two genes (PPP3R1 and PPP3R2, respectively). The CnB1 protein is expressed ubiquitously, whereas the CnB2 protein is specifically expressed in the testes. CnA contains an amino-terminal catalytic domain followed by a CnB-binding domain, a CaM-binding domain, and an autoinhibitory domain. CaN also contains a nuclear localization signal (NLS) in the catalytic domain and a nuclear export signal (NES) in the carboxyl terminus. The autoinhibitory domain of CnA blocks the catalytic site and the NLS [56,62][40][46]. CnB contains four EF-hand Ca2+-binding motifs and an amino-terminal myristylation site. CaN is activated by the increased intracellular Ca2+ concentration in the cell and plays essential roles in multiple signaling processes [63][47]. The binding of Ca2+/CaM to the regulatory domain leads to the attenuation of autoinhibition, followed by dramatic enzymatic activation. Of note, although there are multiple kinases that are regulated by CaM, and CaN is the only phosphatase directly regulated by Ca2+/CaM.5. Mechanisms Regulating Calcineurin Activity
Several proteins have been reported to inhibit CaN, including AKAP79 (A-kinase anchoring protein-79) [69][48], PMCA2 (plasma membrane calcium ATPase 2) [70][49], CHP (calcineurin homologous protein) [71,72][50][51], Cabin/Cain [73[52][53],74], calcipressin/RCAN/DSCR/CSP [75[54][55][56],76,77], and FK506-binding protein (FKBP) 38 [78][57]. Subcellular localization of CaN is regulated by its interaction with AKAP79, a scaffold protein that anchors CaN at distinct subcellular locations, leading to the inhibition of CaN [67,69,79][58][48][59]. In breast cancer cells, PMCA2 interacts with and sequesters CaN in the membrane, and suppresses the activation of the CaN-NFAT pathway [70][49]. CHP competes with CnB to bind to CnA, and inhibits CaN activity [71,72][50][51]. While Cabin1/cain inhibits CaN by interacting with CaN in a phosphorylation-dependent manner through a binding site on CaN, which is distinct from that of the drug–immunophilin complex [73,74][52][53]. Calcipressin/RCAN/DSCR/CSP has also been identified as a CnA-binding protein that inhibits CaN activity [77,80][56][60]. A conserved peptide (FLISPPxSPP) of the calcipressin family is phosphorylated and functions as a binding site for CaN. As the expression of calcipressin is induced by CaN, it functions as a feedback inhibitor of CaN signaling. Calcipressin/RCAN/DSCR/CSP binds CaN at the same site as NFAT and other substrates, with competition for binding between these molecules being a possible regulatory mechanism [81][61]. FKBP38 targets BCL-2 to the mitochondria and inhibits apoptosis. The same protein also binds to and inhibits CaN, even in the absence of FK506 [78][57]. Furthermore, histone H1 inhibits CaMKII and CaN by blocking CaM autophsophorylation [82][62]. CaN is also inactivated by the oxidation of key methionine residues [83,84,85,86][63][64][65][66]. Conversely, CaN is activated by the intramolecular cleavage by two different proteases, caspase 3 and Ca2+-dependent protease calpain [87,88,89][67][68][69].6. Functions of Calcineurin/NFAT
Cyclosporine A and FK506 are well-characterized immunosuppressive agents that prevent organ transplant rejection [93,94,95][70][71][72]. These compounds bind tightly to endogenous cytoplasmic cyclophilin A or FKBP12, respectively. Interestingly, cyclophilin A or FKBP12, in complex with cyclosporine A or FK506, bind to CaN and block the access of the CaN substrate to the active site of the CaN [96][73]. This indicates the possibility that the immunosuppressive effects of these drugs could be, in part, caused by the disruption of CaN functions. Consistent with this view, it has been show that CaN inhibition by cyclosporine A or FK506 delays G1/S progression in various cell types [97,98,99,100][74][75][76][77]. Mechanistically, cyclosporin A induces the expression of the cyclin inhibitor p21 and a reciprocal reduction in cyclins A and E, leading to CDK2 inactivation [101,102][78][79].7. A Therapeutic Perspective for Cancer
Owing to the high frequency of CaN/NFAT activation in cancer and the contribution of these molecules in cancer progression, the CaN/NFAT pathway could be a potential therapeutic target. Indeed, the anticancer effects of CaN inhibitors have been studied extensively in the past. For instance, cyclosporine A or FK506 induced apoptosis and rapid tumor clearance, resulting in the regression of leukemia [160][80]. Cyclosporine A or FK506 also inhibits tumor growth in the bladder and prostate xenografts in vivo [174,178,179][81][82][83]. In addition, cyclosporine A itself is also directly involved in tumor growth, as it increases TGFβ production [180][84], activates Ras [181][85], suppresses PTEN expression, and increases AKT activation [182][86].8. Conclusions
Nuclear Ca2+ is involved in tumor growth and alters the expression of the genes involved in cell proliferation. Furthermore, previous studies have suggested that the modulation of nuclear Ca2+ signaling may be effective in cancer therapy. Activation of CaN and its downstream dephosphorylation has been identified as a mechanism by which nuclear Ca2+ regulates cell proliferation and cell cycle progression. As discussed above, dephosphorylation of proteins by CaN plays an important role in tumor formation and progression. Therefore, in the future, it will be necessary to identify the molecular mechanism of CaN activation and the substrates that promote cancer cell growth. Targeting the interactions of activated CaN with specific substrates in cancer cells, without affecting the normal immune function of CaN, may effectively inhibit the growth of cancer cells, leading to the establishment of new tumor-specific therapies.References
- Berridge, M.J.; Lipp, P.; Bootman, M.D. The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol. 2000, 1, 11–21.
- Patel, S.; Joseph, S.; Thomas, A. Molecular properties of inositol 1,4,5-trisphosphate receptors. Cell Calcium 1999, 25, 247–264.
- Kiselyov, K.; Mignery, G.A.; Zhu, M.X.; Muallem, S. The N-Terminal Domain of the IP3 Receptor Gates Store-Operated hTrp3 Channels. Mol. Cell 1999, 4, 423–429.
- Van Rossum, D.; Patterson, R.L.; Kiselyov, K.; Boehning, D.; Barrow, R.K.; Gill, D.L.; Snyder, S.H. Agonist-induced Ca2+ entry determined by inositol 1,4,5-trisphosphate recognition. Proc. Natl. Acad. Sci. USA 2004, 101, 2323–2327.
- Venkatachalam, K.; Van Rossum, D.B.; Patterson, R.L.; Ma, H.-T.; Gill, D.L. The cellular and molecular basis of store-operated calcium entry. Nat. Cell Biol. 2002, 4, E263–E272.
- Parkash, J.; Asotra, K. Calcium wave signaling in cancer cells. Life Sci. 2010, 87, 587–595.
- McConkey, D.J.; Orrenius, S. The role of calcium in the regulation of apoptosis. Biochem. Biophys. Res. Commun. 1997, 239, 357–366.
- Santella, L. The Role of Calcium in the Cell Cycle: Facts and Hypotheses. Biochem. Biophys. Res. Commun. 1998, 244, 317–324.
- Pinto, M.C.X.; Kihara, A.; Goulart, V.A.; Tonelli, F.M.P.; Gomes, K.N.; Ulrich, H.; Resende, R.R. Calcium signaling and cell proliferation. Cell. Signal. 2015, 27, 2139–2149.
- Smedler, E.; Uhlén, P. Frequency decoding of calcium oscillations. Biochim. Biophys. Acta (BBA) Gen. Subj. 2014, 1840, 964–969.
- Kahl, C.R.; Means, A.R. Regulation of Cell Cycle Progression by Calcium/Calmodulin-Dependent Pathways. Endocr. Rev. 2003, 24, 719–736.
- Whitfield, J.F. Calcium signals and cancer. Crit. Rev. Oncog. 1992, 3, 55–90.
- Cook, S.J.; Lockyer, P.J. Recent advances in Ca2+-dependent Ras regulation and cell proliferation. Cell Calcium 2006, 39, 101–112.
- Morgan, D.O. Cyclin-Dependent Kinases: Engines, Clocks, and Microprocessors. Annu. Rev. Cell Dev. Biol. 1997, 13, 261–291.
- Satyanarayana, A.; Kaldis, P. Mammalian cell-cycle regulation: Several Cdks, numerous cyclins and diverse compensatory mechanisms. Oncogene 2009, 28, 2925–2939.
- Matsushime, H.; Ewen, M.E.; Strom, D.K.; Kato, J.Y.; Hanks, S.K.; Roussel, M.F.; Sherr, C.J. Identification and properties of an atypical catalytic subunit (p34PSK-J3/cdk4) for mammalian D type G1 cyclins. Cell 1992, 71, 323–334.
- Connell-Crowley, L.; Harper, J.W.; Goodrich, D.W. Cyclin D1/Cdk4 regulates retinoblastoma protein-mediated cell cycle arrest by site-specific phosphorylation. Mol. Biol. Cell 1997, 8, 287–301.
- Zarkowska, T.; Mittnacht, S. Differential phosphorylation of the retinoblastoma protein by G1/S cyclin-dependent kinases. J. Biol. Chem. 1997, 272, 12738–12746.
- Bertoli, C.; Skotheim, J.M.; De Bruin, R.A.M. Control of cell cycle transcription during G1 and S phases. Nat. Rev. Mol. Cell Biol. 2013, 14, 518–528.
- Weinberg, R.A. The retinoblastoma protein and cell cycle control. Cell 1995, 81, 323–330.
- Dyson, N. The regulation of E2F by pRB-family proteins. Genes Dev. 1998, 12, 2245–2262.
- Petersen, B.O.; Lukas, J.; Sorensen, C.; Bartek, J.; Helin, K. Phosphorylation of mammalian CDC6 by Cyclin A/CDK2 regulates its subcellular localization. EMBO J. 1999, 18, 396–410.
- Coverley, D.; Pelizon, C.; Trewick, S.; Laskey, R. Chromatin-bound Cdc6 persists in S and G2 phases in human cells, while soluble Cdc6 is destroyed in a cyclin A-cdk2 dependent process. J. Cell Sci. 2000, 113, 1929–1938.
- Yam, C.H.; Fung, T.K.; Poon, R. Cyclin A in cell cycle control and cancer. Cell. Mol. Life Sci. 2002, 59, 1317–1326.
- Stillman, B. Cell Cycle Control of DNA Replication. Science 1996, 274, 1659–1663.
- Kishimoto, T.; Okumura, E. In vivo regulation of the entry into M-phase: Initial activation and nuclear translocation of cyclin B/Cdc2. Prog. Cell Cycle Res. 1997, 3, 241–249.
- Furuno, N.; Elzen, N.D.; Pines, J. Human Cyclin a Is Required for Mitosis until Mid Prophase. J. Cell Biol. 1999, 147, 295–306.
- Boynton, A.L.; Whitfield, J.F.; Isaacs, R.J.; Tremblay, R. The control of human WI-38 cell proliferation by extracellular calcium and its elimination by SV-40 virus-induced proliferative transformation. J. Cell. Physiol. 1977, 92, 241–247.
- Russa, A.D.; Maesawa, C.; Satoh, Y. Spontaneous i oscillations in G1/S phase-synchronized cells. J. Electron. Microsc. 2009, 58, 321–329.
- Chin, D.; Means, A.R. Calmodulin: A prototypical calcium sensor. Trends Cell Biol. 2000, 10, 322–328.
- Colomer, J.; Lopezgirona, A.; Agell, N.; Bachs, O. Calmodulin Regulates the Expression of CDKS, Cyclins and Replicative Enzymes During Proliferative Activation of Human T Lymphocytes. Biochem. Biophys. Res. Commun. 1994, 200, 306–312.
- Rasmussen, C.D.; Means, A.R. Calmodulin is required for cell-cycle progression during G1 and mitosis. EMBO J. 1989, 8, 73–82.
- Choi, J.; Husain, M. Calmodulin-Mediated Cell Cycle Regulation: New Mechanisms for Old Observations. Cell Cycle 2006, 5, 2183–2186.
- Schulman, H.; Hanson, P.I. Multifunctional Ca2+/calmodulin-dependent protein kinase. Neurochem. Res. 1993, 18, 65–77.
- Fujisawa, H. Regulation of the activities of multifunctional Ca2+/calmodulin-dependent protein kinases. J. Biochem. 2001, 129, 193–199.
- Karpurapu, M.; Wang, D.; Van Quyen, D.; Kim, T.K.; Kundumani-Sridharan, V.; Pulusani, S.; Rao, G.N. Cyclin D1 is a bona fide target gene of NFATc1 and is sufficient in the mediation of injury-induced vascular wall remodeling. J. Biol. Chem. 2010, 285, 3510–3523.
- Goshima, T.; Habara, M.; Maeda, K.; Hanaki, S.; Kato, Y.; Shimada, M. Calcineurin regulates cyclin D1 stability through dephosphorylation at T286. Sci. Rep. 2019, 9, 1–11.
- Klee, C.B.; Ren, H.; Wang, X. Regulation of the Calmodulin-stimulated Protein Phosphatase, Calcineurin. J. Biol. Chem. 1998, 273, 13367–13370.
- Hogan, P.G.; Li, H. Calcineurin. Curr. Biol. 2005, 15, R442–R443.
- Li, H.; Rao, A.; Hogan, P.G. Interaction of calcineurin with substrates and targeting proteins. Trends Cell Biol. 2011, 21, 91–103.
- Rusnak, F.; Mertz, P. Calcineurin: Form and Function. Physiol. Rev. 2000, 80, 1483–1521.
- Klee, C.B.; Draetta, G.F.; Hubbard, M.J. Calcineurin. Adv. Enzym. Relat. Areas Mol. Biol. 1988, 61, 149–200.
- Cohen, P.T.; Chen, M.X.; Armstrong, C.G. Novel Protein Phosphatases That May Participate in Cell Signaling. Adv. Pharmacol. 1996, 36, 67–89.
- Goto, S.; Matsukado, Y.; Mihara, Y.; Inoue, N.; Miyamoto, E. Calcineurin in human brain and its relation to extrapyramidal system. Immunohistochemical study on postmortem human brains. Acta Neuropathol. 1986, 72, 150–156.
- Kuno, T.; Mukai, H.; Ito, A.; Chang, C.D.; Kishima, K.; Saito, N.; Tanaka, C. Distinct cellular expression of calcineurin A alpha and A beta in rat brain. J. Neurochem. 1992, 58, 1643–1651.
- Hallhuber, M.; Burkard, N.; Wu, R.; Buch, M.H.; Engelhardt, S.; Hein, L.; Neyses, L.; Schuh, K.; Ritter, O. Inhibition of nuclear import of calcineurin prevents myocardial hypertrophy. Circ. Res. 2006, 99, 626–635.
- Park, Y.-J.; Yoo, S.-A.; Kim, M.; Kim, W.-U. The Role of Calcium–Calcineurin–NFAT Signaling Pathway in Health and Autoimmune Diseases. Front. Immunol. 2020, 11, 195.
- Coghlan, V.M.; Perrino, B.A.; Howard, M.; Langeberg, L.K.; Hicks, J.B.; Gallatin, W.M.; Scott, J.D. Association of Protein Kinase A and Protein Phosphatase 2B with a Common Anchoring Protein. Science 1995, 267, 108–111.
- Baggott, R.R.; Mohamed, T.M.; Oceandy, D.; Holton, M.; Blanc, M.C.; Roux-Soro, S.C.; Brown, S.; Brown, J.E.; Cartwright, E.; Wang, W.; et al. Disruption of the interaction between PMCA2 and calcineurin triggers apoptosis and enhances paclitaxel-induced cytotoxicity in breast cancer cells. Carcinogenesis 2012, 33, 2362–2368.
- Lin, X.; Barber, D.L. A calcineurin homologous protein inhibits GTPase-stimulated Na-H exchange. Proc. Natl. Acad. Sci. USA 1996, 93, 12631–12636.
- Lin, X.; Sikkink, R.A.; Rusnak, F.; Barber, D.L. Inhibition of Calcineurin Phosphatase Activity by a Calcineurin B Homologous Protein. J. Biol. Chem. 1999, 274, 36125–36131.
- Sun, L.; Youn, H.-D.; Loh, C.; Stolow, M.; He, W.; Liu, J.O. Cabin 1, A Negative Regulator for Calcineurin Signaling in T Lymphocytes. Immunity 1998, 8, 703–711.
- Lai, M.M.; Burnett, P.E.; Wolosker, H.; Blackshaw, S.; Snyder, S.H. Cain, A Novel Physiologic Protein Inhibitor of Calcineurin. J. Biol. Chem. 1998, 273, 18325–18331.
- Rothermel, B.; Vega, R.B.; Yang, J.; Wu, H.; Bassel-Duby, R.; Williams, R.S. A Protein Encoded within the Down Syndrome Critical Region Is Enriched in Striated Muscles and Inhibits Calcineurin Signaling. J. Biol. Chem. 2000, 275, 8719–8725.
- Fuentes, J.J.; Genescà, E.; Kingsbury, T.J.; Cunningham, K.W.; Perez-Riba, M.; Estivill, X.; de la Luna, S. DSCR1, overexpressed in Down syndrome, is an inhibitor of calcineurin-mediated signaling pathways. Hum. Mol. Genet. 2000, 9, 1681–1690.
- Kingsbury, T.J.; Cunningham, K.W. A conserved family of calcineurin regulators. Genes Dev. 2000, 14, 1595–1604.
- Shirane, M.; Nakayama, K.I. Inherent calcineurin inhibitor FKBP38 targets Bcl-2 to mitochondria and inhibits apoptosis. Nat. Cell Biol. 2003, 5, 28–37.
- Natarajan, K.; Ness, J.; Wooge, C.H.; Janovick, J.A.; Conn, P.M. Specific Identification and Subcellular Localization of Three Calmodulin-binding Proteins in the Rat Gonadotrope: Spectrin, Caldesmon, and Calcineurin. Biol. Reprod. 1991, 44, 43–52.
- Li, H.; Pink, M.D.; Murphy, J.G.; Stein, A.; Dell’Acqua, M.L.; Hogan, P.G. Balanced interactions of calcineurin with AKAP79 regulate Ca2+–calcineurin–NFAT signaling. Nat. Struct. Mol. Biol. 2012, 19, 337–345.
- Görlach, J.; Fox, D.S.; Cutler, N.S.; Cox, G.M.; Perfect, J.R.; Heitman, J. Identification and characterization of a highly conserved calcineurin binding protein, CBP1/calcipressin, inCryptococcus neoformans. EMBO J. 2000, 19, 3618–3629.
- Martinez-Martinez, S.; Genescà, E.; Rodriguez, A.; Raya, A.; Salichs, E.; Were, F.; Lopez-Maderuelo, M.D.; Redondo, J.M.; de la Luna, S. The RCAN carboxyl end mediates calcineurin docking-dependent inhibition via a site that dictates binding to substrates and regulators. Proc. Natl. Acad. Sci. USA 2009, 106, 6117–6122.
- Rasmussen, C.; Garen, C. Activation of calmodulin-dependent enzymes can be selectively inhibited by histone H1. J. Biol. Chem. 1993, 268, 23788–23791.
- Wang, X.; Culotta, V.C.; Klee, C.B. Superoxide dismutase protects calcineurin from inactivation. Nature 1996, 383, 434–437.
- Sommer, D.; Fakata, K.L.; Swanson, S.A.; Stemmer, P. Modulation of the phosphatase activity of calcineurin by oxidants and antioxidants in vitro. JBIC J. Biol. Inorg. Chem. 2000, 267, 2312–2322.
- Namgaladze, D.; Shcherbyna, I.; Kienhöfer, J.; Hofer, H.W.; Ullrich, V. Superoxide targets calcineurin signaling in vascular endothelium. Biochem. Biophys. Res. Commun. 2005, 334, 1061–1067.
- Zhou, X.; Mester, C.; Stemmer, P.; Reid, G.E. Oxidation-Induced Conformational Changes in Calcineurin Determined by Covalent Labeling and Tandem Mass Spectrometry. Biochemistry 2014, 53, 6754–6765.
- Mukerjeea, N.; McGinnis, K.M.; Gnegy, M.E.; Wang, K.K. Caspase-Mediated Calcineurin Activation Contributes to IL-2 Release during T Cell Activation. Biochem. Biophys. Res. Commun. 2001, 285, 1192–1199.
- Kim, M.-J.; Jo, D.-G.; Hong, G.-S.; Kim, B.J.; Lai, M.; Cho, D.-H.; Kim, K.-W.; Bandyopadhyay, A.; Hong, Y.-M.; Kim, D.H.; et al. Calpain-dependent cleavage of cain/cabin1 activates calcineurin to mediate calcium-triggered cell death. Proc. Natl. Acad. Sci. USA 2002, 99, 9870–9875.
- Wu, H.-Y.; Tomizawa, K.; Oda, Y.; Wei, F.-Y.; Lu, Y.-F.; Matsushita, M.; Li, S.T.; Moriwaki, A.; Matsui, H. Critical Role of Calpain-mediated Cleavage of Calcineurin in Excitotoxic Neurodegeneration. J. Biol. Chem. 2004, 279, 4929–4940.
- Liu, J.; Farmer, J.D.; Lane, W.S.; Friedman, J.; Weissman, I.; Schreiber, S.L. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell 1991, 66, 807–815.
- Shaw, K.T.; Ho, A.M.; Raghavan, A.; Kim, J.; Jain, J.; Park, J.; Sharma, S.; Rao, A.; Hogan, P.G. Immunosuppressive drugs prevent a rapid dephosphorylation of transcription factor NFAT1 in stimulated immune cells. Proc. Natl. Acad. Sci. USA 1995, 92, 11205–11209.
- Hogan, P.G.; Chen, L.; Nardone, J.; Rao, A. Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev. 2003, 17, 2205–2232.
- Roy, J.; Cyert, M.S. Identifying New Substrates and Functions for an Old Enzyme: Calcineurin. Cold Spring Harb. Perspect. Biol. 2019, 12, 3.
- Lally, C.; Healy, E.; Ryan, M.P. Cyclosporine A-induced cell cycle arrest and cell death in renal epithelial cells. Kidney Int. 1999, 56, 1254–1257.
- Schneider, G.; Oswald, F.; Wahl, C.; Greten, F.R.; Adler, G.; Schmid, R.M. Cyclosporine inhibits growth through the activating transcription factor/cAMP-responsive element-binding protein binding site in the cyclin D1 promoter. J. Biol. Chem. 2002, 277, 43599–43607.
- Kahl, C.R.; Means, A.R. Calcineurin Regulates Cyclin D1 Accumulation in Growth-stimulated Fibroblasts. Mol. Biol. Cell 2004, 15, 1833–1842.
- Toyota, N.; Hashimoto, Y.; Matsuo, S.; Kitamura, Y.; Iizuka, H. Effects of FK506 and cyclosporin A on proliferation, histamine release and phenotype of murine mast cells. Arch. Dermatol. Res. 1996, 288, 474–480.
- Khanna, A.K.; Hosenpud, J.D. Cyclosporine Induces The Expression of the Cyclin Inhibitor p21. Transplantation 1999, 67, 1262–1268.
- Tomono, M.; Toyoshima, K.; Ito, M.; Amano, H.; Kiss, Z. Inhibitors of Calcineurin Block Expression of Cyclins A and E Induced by Fibroblast Growth Factor in Swiss 3T3 Fibroblasts. Arch. Biochem. Biophys. 1998, 353, 374–378.
- Medyouf, H.; Alcalde, H.; Berthier, C.; Guillemin, M.C.; dos Santos, N.R.; Janin, A.; Decaudin, D.; De Thé, H.; Ghysdael, J. Targeting calcineurin activation as a therapeutic strategy for T-cell acute lymphoblastic leukemia. Nat. Med. 2007, 13, 736–741.
- Kawahara, T.; Kashiwagi, E.; Ide, H.; Li, Y.; Zheng, Y.; Ishiguro, H.; Miyamoto, H. The role of NFATc1 in prostate cancer progression: Cyclosporine A and tacrolimus inhibit cell proliferation, migration, and invasion. Prostate 2015, 75, 573–584.
- Kawahara, T.; Kashiwagi, E.; Ide, H.; Li, Y.; Zheng, Y.; Miyamoto, Y.; Netto, G.J.; Ishiguro, H.; Miyamoto, H. Cyclosporine A and tacrolimus inhibit bladder cancer growth through down-regulation of NFATc1. Oncotarget 2015, 6, 1582–1593.
- Siamakpour-Reihani, S.; Caster, J.; Bandhu Nepal, D.; Courtwright, A.; Hilliard, E.; Usary, J.; Ketelsen, D.; Darr, D.; Shen, X.J.; Patterson, C.; et al. The Role of Calcineurin/NFAT in SFRP2 Induced Angiogenesis—A Rationale for Breast Cancer Treatment with the Calcineurin Inhibitor Tacrolimus. PLoS ONE 2011, 6, e20412.
- Hojo, M.; Morimoto, T.; Maluccio, M.; Asano, T.; Morimoto, K.; Lagman, M.; Shimbo, T.; Suthanthiran, M. Cyclosporine induces cancer progression by a cell-autonomous mechanism. Nature 1999, 397, 530–534.
- Datta, D.; Contreras, A.; Basu, A.; Dormond, O.; Flynn, E.; Briscoe, D.; Pal, S. Calcineurin Inhibitors Activate the Proto-Oncogene Ras and Promote Protumorigenic Signals in Renal Cancer Cells. Cancer Res. 2009, 69, 8902–8909.
- Han, W.; Ming, M.; He, T.-C.; He, Y.-Y. Immunosuppressive Cyclosporin A Activates AKT in Keratinocytes through PTEN Suppression: Implications In Skin Carcinogenesis. J. Biol. Chem. 2010, 285, 11369–11377.
