Some say that all diseases begin in the gut. Interestingly, this concept is actually quite old, since it is attributed to the Ancient Greek physician Hippocrates, who proposed the hypothesis nearly 2500 years ago. The continuous breakthroughs in modern medicine have transformed our classic understanding of the gastrointestinal tract (GIT) and human health. Although the gut microbiota (GMB) has proven to be a core component of human health under standard metabolic conditions, there is now also a strong link connecting the composition and function of the GMB to the development of numerous diseases, especially the ones of musculoskeletal nature. The symbiotic microbes that reside in the gastrointestinal tract are very sensitive to biochemical stimuli and may respond in many different ways depending on the nature of these biological signals. Certain variables such as nutrition and physical modulation can either enhance or disrupt the equilibrium between the various species of gut microbes.
- osteoarthritis
- gut microbiota
- metabolic syndrome
- systemic inflammation
1. Introduction
2. GBM-Derived Metabolites and Osteoarthritis (OA) Progression
Recent attention has been given to bacterial-derived LPS, specifically, as this microbial protein has been increasingly implicated in inflammatory disorders, namely OA. Researchers have revealed a correlation between elevated levels of circulatory inflammatory biomarkers (including LPS) with the severity of OA, therefore painting GMB-derived metabolites as pathogenic mediators responsible for driving inflammatory musculoskeletal disorders [65,66][17][18]. For instance, an animal study demonstrated that mice on a 28-week high-fat and high-sugar diet developed an obese phenotype and displayed increased cartilage damage, establishing a direct correlation between serum LPS levels and Mankin histological scores [65][17]. In this study the authors also examined GMB composition via 16S sequencing, detecting significant increases in Lactobacillus and Methanobrevibacter bacterial species, which indicated that MS promoted a strong dysbiotic shift in murine GMB with a strong predictive relationship with histological scores. In a similar study, Ulici et al. were able to demonstrate reduced severity of post-traumatic OA in germ-free mice, implying once again a causal role for the GMB in musculoskeletal pathogenesis [67][19]. Most of the animal studies evaluating the impact of GMB were performed on rodents due to the similarity of their GMB to that of the human gut microenvironment [68,69][20][21]. A similar pathogenic process occurs in humans. Dysbiosis of gut microbiome promotes excess porosity in the epithelial barrier of the gut and leakage of microbes and their by-products into the circulation, as shown in Figure 1 [70,71][22][23]. Stress involved in metabolic syndrome and pain involved in OA modulate gut microbiota through release of neurotransmitters and result in increased intestinal permeability [71,72][23][24].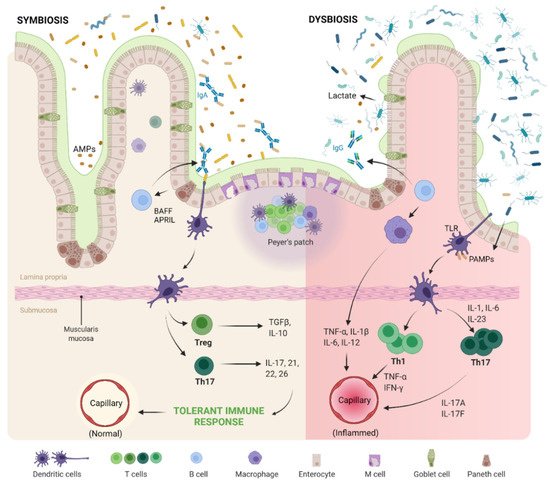
2.1. Gut–Joint Axis Distortion
2.2. Gut–Joint–Brain Axis Distortion
2.3. Evidences on Pathogenesis of OA
References
- Hunter, D.J.; March, L.; Chew, M. Osteoarthritis in 2020 and beyond: A Lancet Commission. Lancet 2020, 396, 1711–1712.
- Cui, A.; Li, H.; Wang, D.; Zhong, J.; Chen, Y.; Lu, H. Global, Regional Prevalence, Incidence and Risk Factors of Knee Osteoarthritis in Population-Based Studies. EClinicalMedicine 2020, 29, 100587.
- Azzini, G.O.M.; Santos, G.S.; Visoni, S.B.C.; Azzini, V.O.M.; Dos Santos, R.G.; Huber, S.C.; Lana, J.F. Metabolic Syndrome and Subchondral Bone Alterations: The Rise of Osteoarthritis—A Review. J. Clin. Orthop. Trauma 2020, 11, S849–S855.
- Chen, D.; Shen, J.; Zhao, W.; Wang, T.; Han, L.; Hamilton, J.L.; Im, H.-J. Osteoarthritis: Toward a Comprehensive Understanding of Pathological Mechanism. Bone Res. 2017, 5, 16044.
- Zhang, Y.; Jordan, J.M. Epidemiology of Osteoarthritis. Clin. Geriatr. Med. 2010, 26, 355–369.
- Lana, J.F.; Macedo, A.; Ingrao, I.L.G.; Huber, S.C.; Santos, G.S.; Santana, M.H.A. Leukocyte-Rich PRP for Knee Osteoarthritis: Current Concepts. J. Clin. Orthop. Trauma 2019, 10, S179–S182.
- Setti, T.; Arab, M.G.L.; Santos, G.S.; Alkass, N.; Andrade, M.A.P.; Lana, J.F.S.D. The Protective Role of Glutathione in Osteoarthritis. J. Clin. Orthop. Trauma 2021, 15, 145–151.
- Alberti, K.G.M.M.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.-C.; James, W.P.T.; Loria, C.M.; Smith, S.C.; et al. Harmonizing the Metabolic Syndrome: A Joint Interim Statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645.
- Li, C.; Xu, M.M.; Wang, K.; Adler, A.J.; Vella, A.T.; Zhou, B. Macrophage Polarization and Meta-Inflammation. Transl. Res. J. Lab. Clin. Med. 2018, 191, 29–44.
- Robinson, W.H.; Lepus, C.M.; Wang, Q.; Raghu, H.; Mao, R.; Lindstrom, T.M.; Sokolove, J. Low-Grade Inflammation as a Key Mediator of the Pathogenesis of Osteoarthritis. Nat. Rev. Rheumatol. 2016, 12, 580–592.
- Kapoor, M.; Martel-Pelletier, J.; Lajeunesse, D.; Pelletier, J.-P.; Fahmi, H. Role of Proinflammatory Cytokines in the Pathophysiology of Osteoarthritis. Nat. Rev. Rheumatol. 2011, 7, 33–42.
- Sellam, J.; Berenbaum, F. The Role of Synovitis in Pathophysiology and Clinical Symptoms of Osteoarthritis. Nat. Rev. Rheumatol. 2010, 6, 625–635.
- Mora, J.C.; Przkora, R.; Cruz-Almeida, Y. Knee Osteoarthritis: Pathophysiology and Current Treatment Modalities. J. Pain Res. 2018, 11, 2189–2196.
- Hafsi, K.; McKay, J.; Li, J.; Lana, J.F.; Macedo, A.; Santos, G.S.; Murrell, W.D. Nutritional, Metabolic and Genetic Considerations to Optimise Regenerative Medicine Outcome for Knee Osteoarthritis. J. Clin. Orthop. Trauma 2019, 10, 2–8.
- Vitetta, L.; Coulson, S.; Linnane, A.W.; Butt, H. The Gastrointestinal Microbiome and Musculoskeletal Diseases: A Beneficial Role for Probiotics and Prebiotics. Pathogens 2013, 2, 606–626.
- Marcum, Z.A.; Hanlon, J.T. Recognizing the Risks of Chronic Nonsteroidal Anti-Inflammatory Drug Use in Older Adults. Ann. Long-Term Care Off. J. Am. Med. Dir. Assoc. 2010, 18, 24.
- Collins, K.; Paul, H.; Reimer, R.; Seerattan, R.; Hart, D.; Herzog, W. Relationship between inflammation, the gut microbiota, and metabolic osteoarthritis development: Studies in a rat model. Osteoarthr. Cartil. 2015, 23, 1989–1998.
- Huang, Z.; Stabler, T.; Pei, F.; Kraus, V. Both systemic and local lipopolysaccharide (LPS) burden are associated with knee OA severity and inflammation. Osteoarthr. Cartil. 2016, 24, 1769–1775.
- Ulici, V.; Kelley, K.; Azcarate-Peril, M.; Cleveland, R.; Sartor, R.; Schwartz, T.; Loeser, R. Osteoarthritis induced by destabilization of the medial meniscus is reduced in germ-free mice. Osteoarthr. Cartil. 2018, 26, 1098–1109.
- Nguyen, T.L.A.; Vieira-Silva, S.; Liston, A.; Raes, J. How informative is the mouse for human gut microbiota research? Dis. Model. Mech. 2015, 8, 1–16.
- Wos-Oxley, M.L.; Bleich, A.; Oxley, A.P.; Kahl, S.; Janus, L.M.; Smoczek, A.; Nahrstedt, H.; Pils, M.C.; Taudien, S.; Platzer, M.; et al. Comparative evaluation of establishing a human gut microbial community within rodent models. Gut Microbes 2012, 3, 234–249.
- Szychlinska, M.A.; Di Rosa, M.; Castorina, A.; Mobasheri, A.; Musumeci, G. A correlation between intestinal microbiota dysbiosis and osteoarthritis. Heliyon 2019, 5, e01134.
- Romero, E.S.; Oliva, E.M.; Pérez, J.A.; Pérez, S.M.; Turroni, S.; Marchese, L.; Villafañe, J. Relationship between the Gut Microbiome and Osteoarthritis Pain: Review of the Literature. Nutrients 2021, 13, 716.
- Collins, S.M.; Surette, M.; Bercik, P. The interplay between the intestinal microbiota and the brain. Nat. Rev. Microbiol. 2012, 10, 735–742.
- Wang, X.; Hunter, D.; Xu, J.; Ding, C. Metabolic triggered inflammation in osteoarthritis. Osteoarthr. Cartil. 2015, 23, 22–30.
- Liu, Y.; Ding, W.; Wang, H.; Dai, L.; Zong, W.; Wang, Y.; Bi, J.; Han, W.; Dong, G. Gut microbiota and obesity-associated osteoarthritis. Osteoarthr. Cartil. 2019, 27, 1257–1265.
- Wang, J.; Gu, X.; Yang, J.; Wei, Y.; Zhao, Y. Gut Microbiota Dysbiosis and Increased Plasma LPS and TMAO Levels in Patients with Preeclampsia. Front. Cell. Infect. Microbiol. 2019, 9, 409.
- Hao, X.; Shang, X.; Liu, J.; Chi, R.; Zhang, J.; Xu, T. The gut microbiota in osteoarthritis: Where do we stand and what can we do? Arthritis Res. Ther. 2021, 23, 42.
- Boutagy, N.E.; McMillan, R.P.; Frisard, M.I.; Hulver, M.W. Metabolic endotoxemia with obesity: Is it real and is it relevant? Biochimie 2016, 124, 11–20.
- Berenbaum, F. Deep phenotyping of osteoarthritis: A step forward. Ann. Rheum. Dis. 2019, 78, 3–5.
- Chadha, R. Revealed aspect of metabolic osteoarthritis. J. Orthop. 2016, 13, 347–351.
- Sellam, J.; Berenbaum, F. Is osteoarthritis a metabolic disease? Jt. Bone Spine 2013, 80, 568–573.
- Berenbaum, F.; Griffin, T.M.; Liu-Bryan, R. Review: Metabolic Regulation of Inflammation in Osteoarthritis. Arthritis Rheumatol. Hoboken NJ 2017, 69, 9–21.
- Favazzo, L.J.; Hendesi, H.; Villani, D.A.; Soniwala, S.; Dar, Q.-A.; Schott, E.M.; Gill, S.R.; Zuscik, M.J. The Gut Microbiome-Joint Connection: Implications in Osteoarthritis. Curr. Opin. Rheumatol. 2020, 32, 92–101.
- Turroni, S.; Pedersini, P.; Villafañe, J.H. The Human Gut Microbiome and Its Relationship with Osteoarthritis Pain. Pain Med. Malden Mass 2021, 22, 1467–1469.
- de Sire, A.; de Sire, R.; Petito, V.; Masi, L.; Cisari, C.; Gasbarrini, A.; Scaldaferri, F.; Invernizzi, M. Gut–Joint Axis: The Role of Physical Exercise on Gut Microbiota Modulation in Older People with Osteoarthritis. Nutrients 2020, 12, 574.
- Gracey, E.; Vereecke, L.; McGovern, D.; Fröhling, M.; Schett, G.; Danese, S.; De Vos, M.; Bosch, F.V.D.; Elewaut, D. Revisiting the gut–joint axis: Links between gut inflammation and spondyloarthritis. Nat. Rev. Rheumatol. 2020, 16, 415–433.
- Zaiss, M.M.; Wu, H.-J.J.; Mauro, D.; Schett, G.; Ciccia, F. The gut–joint axis in rheumatoid arthritis. Nat. Rev. Rheumatol. 2021, 17, 224–237.
- Qaiyum, Z.; Lim, M.; Inman, R.D. The gut-joint axis in spondyloarthritis: Immunological, microbial, and clinical insights. Semin. Immunopathol. 2021, 43, 173–192.
- Huang, Z.; Kraus, V.B. Does lipopolysaccharide-mediated inflammation have a role in OA? Nat Rev Rheumatol. 2016, 12, 123–129.
- Dunn, C.M.; Velasco, C.; Rivas, A.; Andrews, M.; Garman, C.; Jacob, P.B.; Jeffries, M.A. Identification of Cartilage Microbial DNA Signatures and Associations with Knee and Hip Osteoarthritis. Arthritis Rheumatol. 2020, 72, 1111–1122.
- Guss, J.D.; Ziemian, S.N.; Luna, M.; Sandoval, T.N.; Holyoak, D.T.; Guisado, G.G.; Roubert, S.; Callahan, R.L.; Brito, I.L.; van der Meulen, M.C.; et al. The effects of metabolic syndrome, obesity, and the gut microbiome on load-induced osteoarthritis. Osteoarthr. Cartil. 2019, 27, 129–139.
- King, C.; Sibille, K.; Goodin, B.; Cruz-Almeida, Y.; Glover, T.; Bartley, E.; Riley, J.; Herbert, M.; Sotolongo, A.; Schmidt, J.; et al. Experimental pain sensitivity differs as a function of clinical pain severity in symptomatic knee osteoarthritis. Osteoarthr. Cartil. OARS Osteoarthr. Res. Soc. 2013, 21, 1243–1252.
- Boer, C.G.; Radjabzadeh, D.; Medina-Gomez, C.; Garmaeva, S.; Schiphof, D.; Arp, P.; Koet, T.; Kurilshikov, A.; Fu, J.; Ikram, M.A.; et al. Intestinal microbiome composition and its relation to joint pain and inflammation. Nat. Commun. 2019, 10, 4881.
- Mukhtar, K.; Nawaz, H.; Abid, S. Functional gastrointestinal disorders and gut-brain axis: What does the future hold? World J. Gastroenterol. 2019, 25, 552–566.
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids from Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25.
- Boer, C.; Radjabzadeh, D.; Uitterlinden, A.; Kraaij, R.; van Meurs, J. The role of the gut microbiome in osteoarthritis and joint pain. Osteoarthr. Cartil. 2017, 25, S10.
- Coulson, S.; Butt, H.; Vecchio, P.; Gramotnev, H.; Vitetta, L. Green-lipped mussel extract (Perna canaliculus) and glucosamine sulphate in patients with knee osteoarthritis: Therapeutic efficacy and effects on gastrointestinal microbiota profiles. Inflammopharmacology 2013, 21, 79–90.
- Kundu, P.; Blacher, E.; Elinav, E.; Pettersson, S. Our Gut Microbiome: The Evolving Inner Self. Cell 2017, 171, 1481–1493.
