Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Michele Arienzo and Version 4 by Conner Chen.
Rare earth elements (REE) are less than 20% of all elements naturally occurring in the environment. They are defined as a group of 17 elements comprising scandium (Sc), yttrium (Y), and lanthanum (La) elements of group 3B of Periodic Table, and the 14 elements of the lanthanides series, Ce, Pr, Nd, Pm, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, and Lu.
- rare earth elements
- estuary pollution
- bioaccumulation
- ecotoxicity
- environmental tracers
1. Introduction
REE are less than 20% of all elements naturally occurring in the environment [1]. They are defined as a group of 17 elements comprising scandium (Sc), yttrium (Y), and lanthanum (La) elements of group 3B of Periodic Table, and the 14 elements of the lanthanides series, Ce, Pr, Nd, Pm, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, and Lu. Lanthanides have a very similar electronic configuration, with two external s electrons, an internal d electron, and 1 to 14 more internal f electrons, giving rise to a group of very similar elements. Therefore, REE have substantially similar physicochemical properties due to their electronic structure [2]. They have +3 oxidation state, are quite stable, and show contraction, with gradual decline in the ionic radius with increasing atomic number [3]. REE are highly reactive due to their electronic configuration [4]. They have low solubility and easily precipitate or form complexes with hydroxide, carbonate, fluoride, phosphates, or humic and fulvic acids [5][6][7][5,6,7].
All these elements tend to be found together in the same ores [8][9][8,9]. Two further groups are identified: light rare earth elements, LREE from La to Eu, and heavy rare earth elements, HREE, from Gd to Lu and Y [8]. Y, because of its low atomic weight, should be among the LREE, but it is classified with the HREE due to the similarity of its properties, due to its cation Y3+, and radius between Dy and Ho, and deposits with those of this second group [10]. Sc represents the lightest REE, but it is not classified in any of these REE groups because of its absence in the same deposits [10] and because of the difference of its properties with respect to both LREE and HREE [11].
Another classification of the Australian Industry Commission [12], primarily used in mineral extraction terminology, divides REE in three groups: light REE, La, Ce, Pr, Nd, and Pm; medium REE, Sm, Eu, and Gd; heavy REE, Tb, Dy, Ho, Er, Tm, Yb, Lu, Sc, and Y. A mention also deserves the classification of the U.S. Geological Survey (USGS) [13], which is like that of IUPAC [14], considering La, Ce, Pr, Nd, Pr, Sm, Eu, and Gd as LRE, and Tb, Dy, Ho, Er, Tm, Yb, and Lu as HREE.
REE have similar chemical characteristics [15], i.e., most of them exist in the trivalent oxidation state, and their atomic, physical, and chemical properties vary gradually along the series [16]. In the environment, LREE tend to have higher affinities for particles, while HREE are more readily complexed by dissolved ligands [17]. Ce and Eu are atypical and have additional oxidation states, i.e., Ce (VI) and Eu (II) [3].
REE are classified as critical resource materials for high technology industrial applications [18]. REE represent nowadays extremely important ingredients in all high-technology gadgets, and for this reason, they are defined as the vitamins of modern industry [19].
Thus, even though REE in the environment come mainly from geogenic sources, their provenance from high tech devices, agriculture, and medicine is growing steadily. REE have in fact many applications as catalytic converters, Ce; permanent magnets, Pr, Nd, Dy, Ho; batteries, La, Ce, Nd; or magnetic resonance imaging, MRI, agent, Gd; and some REE are also used in the development of drugs for cancer treatment [20][21][22][20,21,22]. They are applied in military defence systems, lighters, flints, fluorescent lamps, high-tech, high-temperature superconductors, information storage, and agricultural products [23][24][25][23,24,25]. They are indispensable in emerging clean energy [26], and hence they are fundamental for the current transition from traditional energy sources to clean energy technologies, wind turbines, electric vehicles, and energy-efficient lighting [27]. The demand for REE from green technologies will reach 51.9 kilotonnes, as rare earth ores in 2030, especially of Nd and Dy [26].
They are in use in agriculture, forestry, animal husbandry, and aquaculture [28] and to increase meat digestibility and quality in diet supplemented with REE-enriched yeast [29]. In China, REE find application as fertilizer supplements to increase yields and crop quality [30]. Lanthanides in the surface soil layer of China reached 100–200 mg/kg [31], with bioaccumulation consequences [32][33][32,33]. Asia—namely, in China—shows the most critical risk of REE pollution level, followed by Europe, Africa, USA, and Australia [34].
Due to such large use, the world consumption of REE in 2020 was 540 kt and is continuously increasing by 6% each year [10][35][10,35].
The two flows of REE, natural and anthropic, may exert cumulative and synergic damaging effects [36], that alter the natural REE distribution [23], especially in natural aquatic systems, disturbing biogeochemical cycles.
REE are recent contaminants, and this limits the knowledge about their environmental fate in terms of bioaccumulation, bioavailability, and toxicity. For this reason, environmental discharge of REE is usually not regulated by governments. Moreover, diffuse REE inputs from the air and water runoff from hard-standing areas hinders the set-up of regulation rules especially in estuaries that receive different input from their catchments [36][37][36,37]. The literature displays [37][38][37,38] that only wastes and treated water from mining activities are embedded in three European regulations, establishing threshold concentrations of REE, EIA directive/EU1452/; Directive/EU 0621/; Directive/EU 1359/ [38]
Thus, REE in aquatic environments, must be considered as ubiquitous contaminants; the presence has already been measured in waters, suspended particles, and sediments of rivers [39], estuaries [40], and oceans [41][42][41,42]. REE have been detected in coastal areas at trace concentrations [43]; however, their harmful ecosystem effects have been only hypothesised [44]. In the marine environment, REE may interact with the resident biota by different pathways. They can bioaccumulate in organisms and be further transferred along the trophic web, resulting in biomagnification or bio-dilution in the upper trophic levels [37]. Environmental concentrations of the fractionated pools of REE, LREE, and HREE, normalized to reference reservoirs, i.e., chondrite, shales, makes it possible to highlight geochemical processes, tracer water masses, and anthropic releases [45][46][47][45,46,47], as is often the case of Sm and Gd showing positive anomalies in many estuaries of the world [48]. Anthropogenic anomalies of Gd, La, and Sa in aqueous samples are often reported [49]. Following anthropogenic Gd, the anomalies of La, Sa, and Eu are reported [50]. The positive Gd anomalies reported for river waters worldwide are caused by the large use of Gd-based contrast agents used in magnetic resonance imaging (MRI) [50]. Sm finds applications in many different areas, from high-strength permanent magnets to control rods in nuclear reactors, and it is used as a catalyst in assisting the decomposition of plastics and the dechlorination of polychlorinated biphenyls [49]. A survey of the anthropogenic dissolved and colloid/nanoparticle-bound Gd and Sm in the Rhine River suggests that while the anthropogenic Gd is not particle-reactive, and hence exclusively present in the truly dissolved REE pool, the anthropogenic Sm is also present in the colloidal/nanoparticulate REE pool [49].
Sholkovitz [51][52][51,52] reports that there is fractionation of REE during the formation of weathering products from parent rocks and that weathered products accumulate REE and tend to be LREE-enriched relative to the parent rock. Fractionation is due, in part, to the formation of more soluble HREE-complexes that are transported away from the weathering zone and to the preferential retention of the LREE by adsorption to or incorporation into secondary minerals. The same author [51][52][51,52] states that the solution pool of REE in river waters is strongly HREE-enriched and is fractionated to the same extent as that of surface seawater. This means that the evolved REE composition of sea water is coupled to chemical weathering on the continents and reactions in estuaries. A particular behaviour is shown by Ce and Eu having valency of +2 besides +3, and +4 as well as +3, respectively influencing the solubility and stability of compounds and hence of anomalies in the REE patterns. It is known that most waters show negative Ce and Eu anomalies [16].
2. The Natural Abundance
The discovery of REE occurred in 1787, but it was not published by Arrhenius until the following year [53]. Their magnetic, luminescent, and electrochemical value has been estimated only few decades ago [54]. The “rare” definition does not reflect their abundance in the environment, which is higher than those of gold or copper, Figure 1, but the form in which they occur, dispersed in ores instead of in the native form of aggregates or nuggets [55][56][55,56].
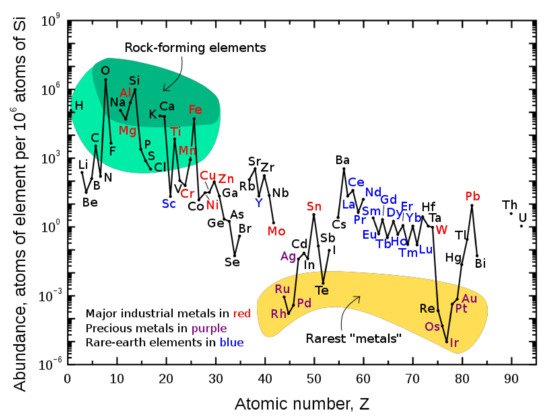

REE are widely distributed in the Earth’s crust in average concentrations ranging from 150 to 220 mg/kg [58][57], which is significantly higher than other commonly exploited elements and much higher than their respective chondritic abundances [59][58].
The mean total crustal abundance of REE is 169 mg/kg, and LREE, La to Gd, are 137.8 mg/kg, far higher than HREE, 31.34 mg/kg. The most abundant REE in the Earth crust are Ce and La, 63 mg/kg and 31 mg/kg, a presence richer than those of Cu, 28 mg/kg, and Pb, 17 mg/kg [60][59]. The rarest ones are Tm and Lu, with levels of 0.30 mg/kg and 0.31 mg/kg, respectively, higher than those of Au, 0.0015 mg/kg, Ag, 0.053 mg/kg, and platinum group elements [60][59]. According to the Oddo–Harkin rule, the REE abundance is greater for those with an even atomic number compared with those with an odd atomic number, and abundance decreases through the lanthanide series [3].
Currently, more than 200 REE-bearing minerals have been identified [56][61][56,61]. Typical existence of REE is in granites, pegmatites, carbonatites, and perovskites [59][58]. They are usually enriched with phosphate-minerals such as apatite, monazite, bastnaesite, and others [59][58]. REE minerals are monazite, bastnaesite, loparite, and the lateritic ion-adsorption clays with high grades of LREE, La–Eu, or ceritic earths and xenotime which contains higher grades of heavy rare earth elements, HREE, Gd–Lu or ytter earths [59][58].
Zhou et al. [54] reported that in the world there are 178 deposits with REE totalling 478 megaton, Mt, as rare earth ores. A survey of 2018 estimates that overall world reserve as metal is 140 Mt, of which 55 are in China, 22 in Brazil, 19 in the Commonwealth of Independent States, and 13 Mt in the USA [13]. Thus, China holds 39% of the overall reserve and produces 100 Kt per year of REE, or 90% in 2010 [62]. These resources could sustain global REE production for more than a hundred years [54]. Moreover, China developed leading processing technologies and production facilities for REE exploitation.
All REE occur in nature but not in pure metal form. Promethium, the rarest, only occurs in trace quantities in natural materials, as it has no long-lived or stable isotopes [63]. LRE resources are found mainly in monazite minerals in northern China, with the Bayan Obo mine in Inner Mongolia being the world’s largest REE mine in operation. HREE resources are enriched in ion adsorption deposits in southern China, where Jiangxi and Guangdong provinces hold almost 70% of the total deposits [64]. Many processing steps are involved in REE manufacturing, making their separation very complex [65].
3. The Sources of REE Contamination
There are several REE sources for estuary aquatic environments that can be natural or anthropic. REE can come from rock and soil erosion processes and wind driven transported to open sea [66]. Post-consumer REE products, waste electronic and electrical equipment (WEEE), once reaching the landfills become an incredible source of REE for oceans [67]. Some of these devices, such as computer screens, can contain up to 72% of Eu in a liquid phase [2][68][2,68]. Thus, recycling plants for WEEE and other REE-containing wastes such as fluorescent lamps, which are common in several countries, including China, India, Pakistan, Nigeria, Ghana, and Vietnam, represent a serious threat [69]. One of the main sources of REE for aquatic systems is the discharge of REE-contaminated wastewater [70], thus representing a great threat for coastal environments via runoff and leaching.
