The microbiome, as a community of microorganisms and their structural elements, genomes, metabolites/signal molecules, has been shown to play an important role in human health, with significant beneficial applications for gut health. Skin microbiome has emerged as a new field with high potential to develop disruptive solutions to manage skin health and disease. Despite an incomplete toolbox for skin microbiome analyses, much progress has been made towards functional dissection of microbiomes and host-microbiome interactions. A standardized and robust investigation of the skin microbiome is necessary to provide accurate microbial information and set the base for a successful translation of innovations in the dermo-cosmetic field.
- skin health
- microbiome
- postbiotics
- microbiome metabolites
- cosmetic
- microbiome data
- methodology harmonization
1. Introduction
2. Microbiome-Based Cosmetic Solutions and Technologies
Many of the skin conditions are multifactorial, however, the microbiome is a key factor in skin disorders. The interplay between the microbiome and the skin is key for its homeostasis health. Intervening and finely modulating the microbiome to correct skin conditions described above is a rising field of research. These interventions are mainly realized by prebiotics, postbiotics, and probiotics, as well as microbiota transplant. The latter is still in its infancy phase for the skin. In cosmetic/dermatology applications, a focus concentrates on the first three paths. The microbiome has been extensively studied and reported in the field of nutrition [5]. Although some definitions exist on the World Health Organization level, there are currently no available international guidelines regarding the definitions or terminologies applicable for cosmetic ingredients that work with the skin’s microbiome. Current definitions consider probiotics to be living microorganisms that must be ingested in a sufficient amount to have a positive effect on health that is not limited to the nutritional effects alone [6][7][8]. Prebiotics are a food ingredient that results in specific changes in the composition and/or activity of the gastrointestinal microbiota, thus conferring benefit(s) upon the host’s health [5]. Very recently, the International Scientific Association of Probiotics and Prebiotics (ISAPP) defined the scope of postbiotics as a “preparation of inanimate microorganisms and/or their components that confers a health benefit on the host”. Postbiotics could be intentionally inactivated microbial cells with or without metabolites, or cell components that contribute to establishing host health benefits. The gut is not the only site of action of postbiotics. They could also be administered on a host surface, such as in the oral cavity or on the skin [9]. The topic of the cosmetic microbiome was taken up in 2018 by the International Cooperation on Cosmetics Regulation (ICCR), a voluntary international group of cosmetic regulatory authorities and cosmetic industry trade associations from Brazil, Canada, Chinese Taipei, the European Union, Japan, the Republic of Korea, and the United States. They considered that new technologies exploring the relationship between the human microbiome and healthy skin were an area of increasing interest and the safety, quality, regulation, and potential development of international guidelines for products arising from these technologies would be a worthwhile topic for the ICCR. In 2020 they developed a set of categories and descriptors that could be used to group and categorize microbiome-related products, their ingredients, and other relevant approaches, in a cosmetic/skin-relevant context [10]. These ingredients were divided into two main categories based on viability: viable (live or dormant)—encompassing only probiotics (based on biological origin), and non-viable ingredients. The non-viable ingredients were further divided into two sub-categories; prebiotics (by their intended action on the skin microbiota) and postbiotics (based on their biological origin) (Table 1).| Postbiotic (Including Probiotic Fraction or Extract) | Prebiotic |
|---|---|
| Non-viable ingredients comprised of inactivated microorganisms and/or soluble factors (products or metabolic by-products) released by live or inactivated microorganisms, added to a cosmetic product to achieve a cosmetic benefit at the application site, either directly or via an effect on the existing microbiota. Categories: 1/Ferments, lysates, extracts, filtrates, 2/Non-viable microorganisms (inactivated/heat-killed), 3/Metabolic products/by-products (isolated) |
Non-viable ingredients are added to a cosmetic product to be actively used as nutrients by the microbiota of the application site to achieve a cosmetic benefit. Examples: ingredients such as fibers, sugars, minerals, but also complex biological mixtures/extracts, etc. |
“Ferments, lysates, extracts, filtrates or any combination of these ingredients that are not living but which have been obtained by means of probiotic bacteria (Bacillus, Bifidobacterium, Lactobacillus, Lactococcus, Vitreoscilla, Streptococcus thermophilus, Leuconostoc) or fungi used primarily as fermentation facilitators (Saccharomyces, Candida bombicola, Kloeckera, Hansenula-Pichia, Aspergillus)”:“Non-viable microorganisms (inactivated/heat-killed), mostly lactic-acid forming bacteria: Enterococcus faecalis, Lactobacillus (paracasei, casei, acidophilus), Lactococcus, or Vitreoscilla filiform”.“Metabolic products/by-products (isolated) including bacteriocin extract, ectoin, succinic acid, lactic acid, hydrolyzed yogurt protein, sodium hyaluronate, and milk proteins” [10].3. Future Implications/Outlook
Postbiotics, including probiotic fractions and effector molecules, are solutions already used in the dermo-cosmetic field; however, the ambition for the cosmetic industry is to add live probiotics to cosmetic formulas with the expectation of potentially higher performance that would probably be driven by a dialogue between added living-microbes and host cells.4. Conclusions
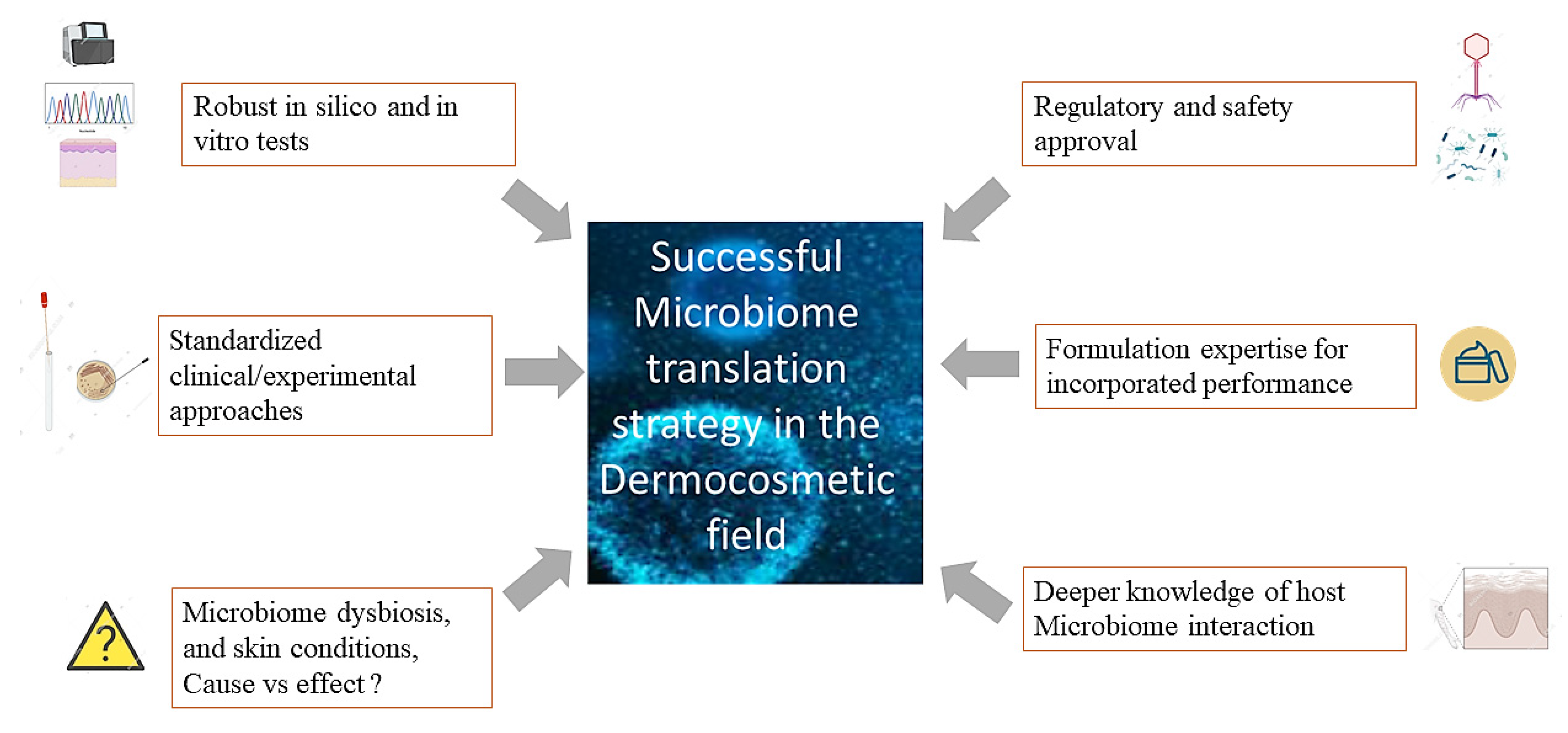
Research is at the dawn of a «new generation» cosmetic that will use the skin’s microbiome to provide lasting products with new efficient performance. To bring to light this rising cosmetics category that harnesses the potential of the cutaneous microbiome, it is essential to dissect the dynamic interactions existing between microorganisms and the interplay host/Microbiome. Researchers would also need to understand the regulatory/safety framework to translate these innovations (Figure 1). However, only rigorous and unbiased experimental approaches considering the specificity of the skin-microbiome environment can be applied. This discovery will be made possible by coupling multi-omics technologies, statistical data mining, and representative 3D skin models. These approaches may provide the opportunity to establish microbiome/skin condition causality and, subsequently, cosmetic solutions. The consideration of subtle regulatory environments and country-specificities will also be of high concern.
References
- Blaser, M.J.; Cardon, Z.G.; Cho, M.K.; Dangl, J.L.; Donohue, T.J.; Green, J.L.; Knight, R.; Maxon, M.E.; Northen, T.R.; Pollard, K.S.; et al. Toward a Predictive Understanding of Earth’s Microbiomes to Address 21st Century Challenges. mBio 2016, 7, e00714-16.
- Brody, H. The gut microbiome. Nature 2020, 577, S5.
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506.
- Grice, E.A. The skin microbiome: Potential for novel diagnostic and therapeutic approaches to cutaneous disease. Semin. Cutan. Med. Surg. 2014, 33, 98–103.
- Mills, S.; Stanton, C.; Lane, J.A.; Smith, G.J.; Ross, R.P. Precision Nutrition and the Microbiome, Part I: Current State of the Science. Nutrients 2019, 11, 923.
- Guarner, F.; Schaafsma, G.J. Probiotics. Int. J. Food Microbiol. 1998, 39, 237–238.
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514.
- Wieërs, G.; Belkhir, L.; Enaud, R.; Leclercq, S.; De Foy, J.-M.P.; Dequenne, I.; De Timary, P.; Cani, P.D. How Probiotics Affect the Microbiota. Front. Cell. Infect. Microbiol. 2020, 9, 454.
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667.
- Report of the ICCR: ICCR Microbiome and Cosmetics—Survey of Products Ingredients Terminologies and Regulatory Approaches. 2021. Available online: https://www.iccr-cosmetics.org/topics-documents/14-microbiome (accessed on 25 November 2021).
- ISO. ISO 17516:2014 Cosmetics—Microbiology—Microbiological Limits; ISO: Geneva, Switzerland, 2014.