Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 3 by Vicky Zhou and Version 2 by Vicky Zhou.
Plant invasion is significantly affected by environmental factors in the recipient habitats and affects the stability and sustainable development of society. The invasiveness of alien plants may be increased by anthropogenic-mediated disturbances, such as fluctuations in nutrients caused by excessive emissions of nitrogen (N) and phosphorus (P).
- plant invasion
- eutrophication
- biogeochemical cycle
- nitrogen
- phosphorus
1. Introduction
Nitrogen (N) and phosphorus (P) are the most important nutrients for plant growth [1]. In plants, N mainly contributes to the biosynthesis of amino acids, proteins, and other macromolecular bioactive substances and is widely involved in various life activities [2]. Similarly, P plays a critical role in the construction of the nucleic acid structure and energy transformation in plants [3]. Maintaining the stability of the biogeochemical cycle of N and P is therefore of great significance to entire ecosystems. However, anthropogenic-mediated disturbances of N and P can have a major influence on the structure and functioning of ecosystems; for example, excessive emissions of N and P change the element compositions and stoichiometries in regional ecosystems and the relationships between species composition and ecological processes [4][5][6]. At the same time, invasive alien species (IAS) also pose a serious threaten to ecosystems, which may cause disturbances in biogenic element cycles, reduced biodiversity, ecosystem degradation, and even destruction of the original ecosystem [7]. As the second greatest threat to biodiversity after habitat fragmentation, invasive alien plants are not dominant competitors in their natural systems but competitively exclude their new neighbors. For example, Centaurea diffusa, a Eurasian plant, has no impact on 32P uptake by other Eurasian species. However, it significantly decreases the 32P absorption of all North American grass species, due to its root activity and chemically mediated effects. On the other hand, no inhibitive effect was found on 32P uptake of C. diffusa by North American grasses, while all Eurasian species showed a great negative influence on 32P uptake by C. diffusa [8].
The composition of plant communities can be altered by the elemental compositions of their habitats [9]. Eutrophication is broadly considered a serious threat to biodiversity conservation and ecosystem functions. It is closely related to plant invasion and expansion. Thus, plant invasion may be triggered by the nutritional conditions in the recipient environment. Some researchers used molecular techniques to survey the phytogeography of two Phragmites spp., Phragmites australis and Phragmites mauritianus, and found that three haplotypes in these species are native, and both species have high genetic diversity. Meanwhile, there was no evidence of recent non-native haplotype invasion in the native region. Therefore, they suggested that the expansion of these two Phragmites species was most likely led by anthropogenic disturbance in the environment [10].
The competitive performance of invasive alien plants is often habitat dependent [11]. That is to say, resource availability in the habitat is a critical factor determining community susceptibility to alien plant invasion. It also determines the ability of alien plants to invade a particular habitat [12]. Among various factors, the coupling effect of N and P dictates the success of alien species invasion [13]. High N and P contents may promote invasion rather than being a consequence of invasion [14]. There is a positive and synergetic effect of N and P on the invasiveness of opportunistic alien species (Figure 1) [4].

Figure 1. Spread of Alternanthera philoxeroides Griseb in eutrophic wetland. Photos were taken in a eutrophic pool invaded by A. philoxeroides Griseb next to Huilong reservoir, Zhenjiang City, Jiangsu Province, China (119°45′ E; 32°15′ N) in 2018. These pictures show spread of A. philoxeroides Griseb over 3 months (9 July to 9 October 2018).
Table 1. Interactions between invasive alien plants and N and P.
| Interactions between Invasive Alien Plants and N and P | References | ||
|---|---|---|---|
| Effects of plant invasion on the N and P pool | N and P pool in invasive alien plants | Higher aboveground N and P accumulation than in native plants | [6][15][16][17][18][19] |
| Larger N investment in photosynthetic production | [20][21][22][23] | ||
| Decreased N allocation to defend structures | [20] | ||
| N and P pool in invaded soil | Increased NH4+ content in soil | [24][25] | |
| Increased total N and P content in soil | [6][26][27] | ||
| Promotion of N and P mineralization and acceleration of N and P cycles | [28][29][30][31][32] | ||
| Mechanisms of plant invasion promoted by N and P | High N and P tolerance hypothesis | [33] | |
| Growth rate hypothesis | [12][13][34][35][36] | ||
| Biomass allocation hypothesis | [37][38] | ||
| Enemy release hypothesis | [39][40][41] | ||
2. Interaction Mechanism between N and P and Invasive Alien Plants
2.1. Influence of Invasive Alien Plants on N and P Pool in Soil and Plants
The success of invasion is mainly the result of the soil status or growing environment of invasive alien plants. Reports have suggested that differences in element compositions and stoichiometries in soils are mainly caused by the success of invasive species in invaded regions [42][43][44][45][46][47]. For example, Hu et al. (2019) reported similar results, showing that NH4+ concentration in soil invaded by Chromolaena odorata was 1.43 times that of native soil [24], and the NH4+ concentration of soil invaded by Ageratina adenophora was 1.56–2.10 times that of native soil [25]. Moreover, soil function may also show some alterations at an early stage of invasion [48]. The differences in soil properties and functioning may point toward the contributions of root exudates [49][50][51] and high productivity litter [26] and their associated spatial variability. For example, compared with the outside areas of the crown canopy of invasive alien plants, the physical and chemical properties of soil under the crown canopy of invasive species were found to be significantly different, although it was only a few meters away [6][52]. Similarly, various other conditions can be advantageous to invasive alien plants over native plants in the acquisition of resources.
Invading plants transform pools and fluxes of N and P in the soil by accelerating N and P cycles in the ecosystem (Figure 2) [28][29][30].
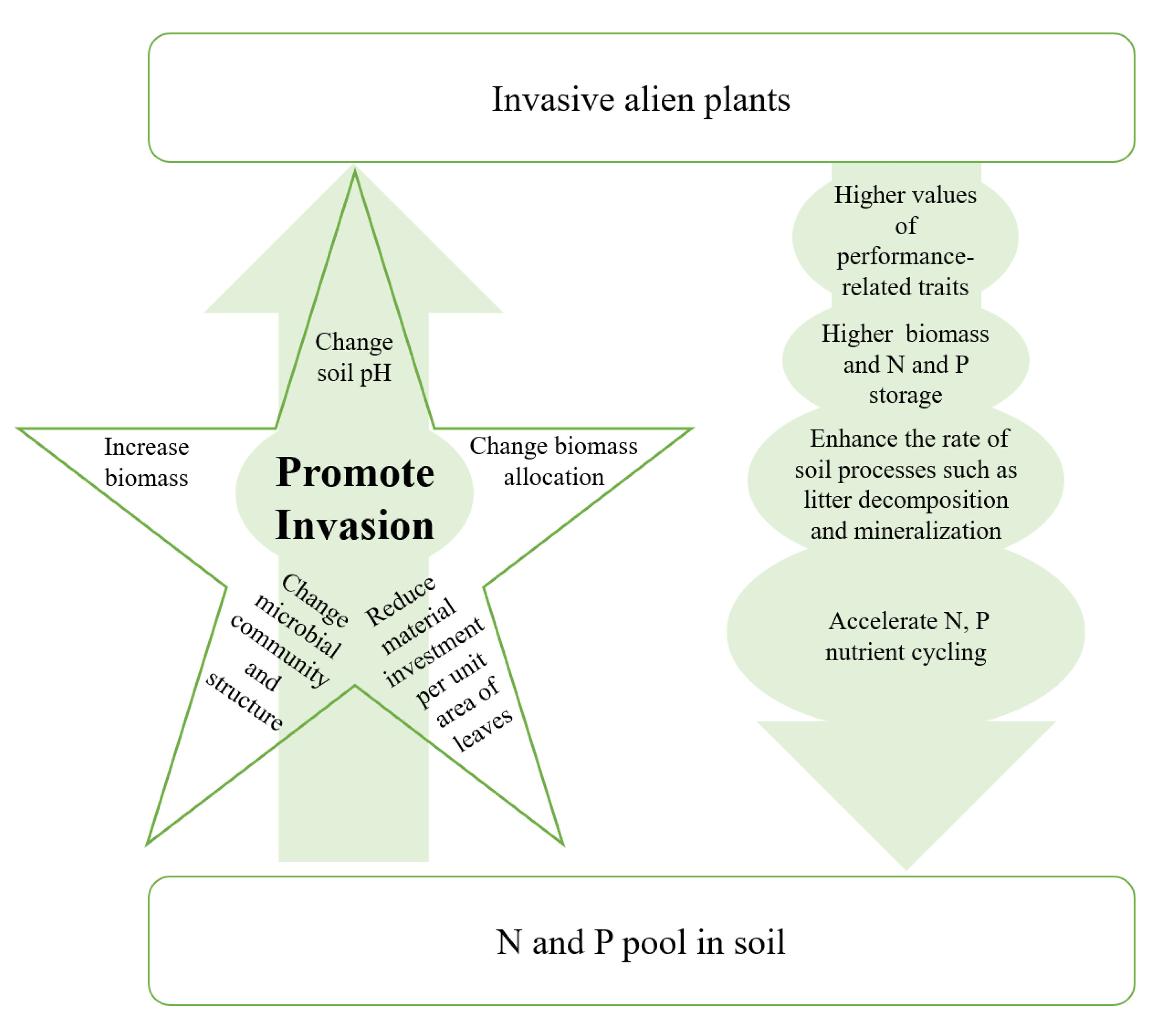

Figure 2. Interaction processes between invasive alien plants and N and P. Eco-mechanisms through which N and P promote plant invasion (left) and how plant invasion intensifies N and P enrichment (right) are shown.
2.2. The Influence of Nutrient Fluctuation Caused by N and P on the Invasiveness of Alien Plants
2.2.1. N and P Mediated Ecological Strategy and Interactions between Native and Invasive Alien Plants
There are differences in plant traits, ecological strategies, and responses to environmental nutrient conditions between invasive and native plants. For example, Dalle Fratte et al. (2019) conducted a study on plant traits in Northern Italy and showed that, due to increased soil N and P loadings in Southern Europe, plant communities are gradually shifting from “slow and conservative” to “fast and acquisitive” species, which may cause invasion by subtropical invasive alien plants and put a strain on biodiversity at the local scale [53]. Previous studies also indicate that invasive alien plants may have different N nutrition strategies compared with native plants. Invasive alien plants such as Bidens pilosa, Microstegium vimineum, and Mikania micrantha prefer to consume nitrate over ammonium [39][42][54]. This “preference” may contribute to its competition with native plants in some nitrate-rich habitats. By contrast, some invasive alien plants are reported to prefer ammonium. The African grass Andropogon gayanus was found to directly alter the understory structure of oligotrophic savannas in tropical Australia, which was attributed to the grass accelerating the ammoniation process and increasing soil ammonium availability to four times that of native plant soil, with a more than six times higher uptake rate of ammonium than native plants [55].
2.2.2. Mechanisms of N and P Promote Alien Plant Invasion
The occurrence of invasion and the growth environment also determine the allocation of resources for the functioning and existence of plants. (1) In a highly N and P polluted environment (e.g., eutrophication), invasive alien plants were found to be more tolerant than most species [33], as they increase N allocation to photosynthetic activity and promote photosynthetic tissue growth. (2) As a response to the growth rate hypothesis, plants demand more rRNA and ribosomes for increased protein synthesis. This requires more phosphorus [13][35] to contribute to higher net primary productivity (NPP) [36], and plants exhibit an increase in overall biomass [12][34]. As shown by Broadbent et al. (2018), under high N conditions, the invasiveness of Agrostis capillaris was significantly increased, and a largely negative impact on native species growth was observed: the biomass of native plants was decreased by half, and total N content in tissues was decreased by up to 75% [27]. Hence, as the fluctuating resource hypothesis describes, those plant communities, which are more sensitive to N and P fluctuations, are more vulnerable to plant invasion [37][38][56]. (3) Additionally, as a response to environmental changes, invasion plant biomass allocation also dictates the changes in N and P levels [40], and this is known as plastic response to difference in resource availability.2.2.3. Strategies to Control Alien Plant Invasion
With ongoing deposition, N would cease to be a limiting nutrient for primary productivity; however, increased N bioavailability might result in a higher P requirement [57][58][59][60]. The accelerated growth of the tested invasive alien plants was found to be closely related to increased leaf P [57][61]. At the same time, this invasive species could selectively utilize insoluble P (Al-P) by enhancing root biomass in an environment with enriched N. A high N concentration in the soil might mean that the N demands are met for both invasive alien plants and native plants after P addition. However, with an even higher N and P demand than that of invasive species (such as Solidago canadensis), native species growth was promoted under N and P enrichment. More nutrients were allocated aboveground, and the growth of invasive alien plants was inhibited through shading. This is an argument in favor of the resource ratio hypothesis [62][63], which holds that plant distributions are determined by the availability of the resource that is most limiting, and resource demands vary among species, which in turn determines the competition effects. Altering the N and P ratio might be an alternative strategy to decrease invasive species competitiveness [61][62].3. Conclusions and Prospects
Excessive emissions of N and P induced by human activity can reduce the resistance of native plants to invasive species. Once we understand their relationship more thoroughly, nutrient element management could serve as “chemotherapy” for invaded habitats contaminated with N and P, for instance, by artificially enhancing nutrient stresses (such as N and P) in ways that have negative influence on invasive instead of native species in a community. In most reports, there is mutual promotion between plant invasion and increased N and P. In other words, resource fluctuations resulting from N and P emissions provide more opportunities and competitiveness for the invasion of alien plants. At the same time, the biogeochemical cycles of N and P are promoted because of their efficient and higher utilization and release rates by invasive alien plants. However, there is no consensus on whether the elemental compositions of invasive species are different from those of natives. Quantitative research comparing the N and P contents of plant, litter, and soil element contents between native plants and invaders in a global context is lacking. Thus, we should further investigate the role of N and P biogeochemical behavior in native and invasive species and the soil in plant–soil ecosystems.References
- Güsewell, S. N: P ratios in terrestrial plants: Variation and functional significance. New Phytol. 2004, 164, 243–266.
- Leghari, S.J.; Wahocho, N.A.; Laghari, G.M.; HafeezLaghari, A.; MustafaBhabhan, G.; HussainTalpur, K.; Bhutto, T.A.; Wahocho, S.A.; Lashari, A.A. Role of nitrogen for plant growth and development: A review. Adv. Environ. Biol. 2016, 10, 209–219.
- Sharma, L.K.; Zaeen, A.A.; Bali, S.K.; Dwyer, J.D. Improving nitrogen and phosphorus efficiency for optimal plant growth and yield. In New Visions in Plant Science; IntechOpen: London, UK, 2017; pp. 13–40.
- Vieira, R.; Pinto, I.S.; Arenas, F. The role of nutrient enrichment in the invasion process in intertidal rock pools. Hydrobiologia 2017, 797, 183–198.
- Li, J.; Leng, Z.; Wu, Y.; Du, Y.; Dai, Z.; Biswas, A.; Zheng, X.; Li, G.; Mahmoud Ek Jia, H.; Du, D. Interactions between invasive plants and heavy metal stresses: A review. J. Plant Ecol. 2021.
- Sardans, J.; Bartrons, M.; Margalef, O.; Gargallo-Garriga, A.; Janssens, I.A.; Ciais, P.; Obersteiner, M.; Sigurdsson, B.D.; Chen, H.Y.H.; Penuelas, J. Plant invasion is associated with higher plant-soil nutrient concentrations in nutrient-poor environments. Glob. Change Biol. 2017, 23, 1282–1291.
- Biederman, L.; Mortensen, B.; Fay, P.; Hagenah, N.; Knops, J.; La Pierre, K.; Laungani, R.; Lind, E.; McCulley, R.; Power, S.; et al. Nutrient addition shifts plant community composition towards earlier flowering species in some prairie ecoregions in the US Central Plains. PLoS ONE 2017, 12, e0178440.
- Mooney, H.A.; Mack, R.; McNeely, J.A.; McNeely, J.A.; Neville, L.E.; Schei, P.J.; Waage, J.K. Invasive Alien Species: A New Synthesis; Island Press: Washington, DC, USA, 2005.
- Callaway, R.M.; Aschehoug, E.T. Invasive plants versus their new and old neighbors: A mechanism for exotic invasion. Science 2000, 290, 521–523.
- Canavan, K.; Paterson, I.D.; Lambertini, C.; Hill, M.P. Expansive reed populations-alien invasion or disturbed wetlands? Aob Plants 2018, 10, ply014.
- Zhang, H.; Chang, R.; Guo, X.; Liang, X.; Wang, R.; Liu, J. Shifts in growth and competitive dominance of the invasive plant Alternanthera philoxeroides under different nitrogen and phosphorus supply. Environ. Exp. Bot. 2017, 135, 118–125.
- González, A.L.; Kominoski, J.S.; Danger, M.; Ishida, S.; Iwai, N.; Rubach, A. Can ecological stoichiometry help explain patterns of biological invasions? Oikos 2010, 119, 779–790.
- Xu, X.; Yang, L.; Huang, X.; Li, Z.; Yu, D. Water brownification may not promote invasions of submerged non-native macrophytes. Hydrobiologia 2018, 817, 215–225.
- Lee, M.R.; Bernhardt, E.S.; van Bodegom, P.M.; Cornelissen, J.H.C.; Kattge, J.; Laughlin, D.C.; Niinemets, U.; Penuelas, J.; Reich, P.B.; Yguel, B.; et al. Invasive species’ leaf traits and dissimilarity from natives shape their impact on nitrogen cycling: A meta-analysis. New Phytol. 2017, 213, 128–139.
- Ordonez, A.; Han, O. Do alien plant species profit more from high resource supply than natives? A trait-based analysis. Glob. Ecol. Biogeogr. 2013, 22, 648–658.
- Ordonez, A.; Wright, I.J.; Olff, H. Functional differences between native and alien species: A global-scale comparison. Funct. Ecol. 2010, 24, 1353–1361.
- Leishman, M.R.; Haslehurst, T.; Ares, A.; Baruch, Z. Leaf trait relationships of native and invasive plants: Community- and global-scale comparisons. New Phytol. 2007, 176, 635–643.
- Baruch, Z.; Goldstein, G. Leaf construction cost, nutrient concentration, and net CO2 assimilation of native and invasive species in Hawaii. Oecologia 1999, 121, 183–192.
- Liu, M.-C.; Kong, D.-L.; Lu, X.-R.; Huang, K.; Wang, S.; Wang, W.-B.; Qu, B.; Feng, Y.-L. Higher photosynthesis, nutrient- and energy-use efficiencies contribute to invasiveness of exotic plants in a nutrient poor habitat in northeast China. Physiol. Plant. 2017, 160, 373–382.
- Feng, Y.L.; Lei, Y.B.; Wang, R.F.; Callaway, R.M.; Valientebanuet, A.; Inderjit Li, Y.P.; Zheng, Y.L. Evolutionary tradeoffs for nitrogen allocation to photosynthesis versus cell walls in an invasive plant. Proc. Natl. Acad. Sci. USA 2009, 106, 1853–1856.
- Feng, Y.L.; Li, Y.P.; Wang, R.F.; Callaway, R.M.; Valiente-Banuet, A. A quicker return energy-use strategy by populations of a subtropical invader in the non-native range: A potential mechanism for the evolution of increased competitive ability. J. Ecol. 2011, 99, 1116–1123.
- Heberling, J.M.; Fridley, J.D. Resource-use strategies of native and invasive plants in Eastern North American forests. New Phytol. 2013, 200, 523–533.
- Heberling, J.M.; Fridley, J.D. Invaders do not require high resource levels to maintain physiological advantages in a temperate deciduous forest. Ecology 2016, 97, 874.
- Hu, C.C.; Lei, Y.B.; Tan, Y.H.; Sun, X.C.; Xu, H.; Liu, C.Q.; Liu, X.Y. Plant nitrogen and phosphorus utilization under invasive pressure in a montane ecosystem of tropical China. J. Ecol. 2019, 107, 372–386.
- Niu, H.B.; Liu, W.X.; Wan, F.H.; Liu, B. An invasive aster (Ageratina adenophora) invades and dominates forest understories in China: Altered soil microbial communities facilitate the invader and inhibit natives. Plant Soil 2007, 294, 73–85.
- Meyerson, L.A.; Saltonstall, K.; Windham, L.; Kiviat, E.; Findlay, S.; Kreeger, D.A.; Weinstein, M.P. A comparison of Phragmites australis in freshwater and brackish marsh environments in North America. Wetl. Ecol. Manag. 2000, 8, 89–103.
- Broadbent, A.; Stevens, C.J.; Peltzer, D.A.; Ostle, N.J.; Orwin, K.H. Belowground competition drives invasive plant impact on native species regardless of nitrogen availability. Oecologia 2018, 186, 577–587.
- Castro-Diez, P.; Godoy, O.; Alonso, A.; Gallardo, A.; Saldana, A. What explains variation in the impacts of exotic plant invasions on the nitrogen cycle? A meta-analysis. Ecol. Lett. 2014, 17, 1–12.
- Vilà, M.; Espinar, J.L.; Hejda, M.; Hulme, P.E.; Jarosik, V.; Maron, J.L.; Pergl, J.; Schaffner, U.; Sun, Y.; Pysek, P. Ecological impacts of invasive alien plants: A meta-analysis of their effects on species, communities and ecosystems. Ecol. Lett. 2011, 14, 702–708.
- Liao, C.; Peng, R.; Luo, Y.; Zhou, X.; Wu, X.; Fang, C.; Chen, J.; Li, B. Altered ecosystem carbon and nitrogen cycles by plant invasion: A meta-analysis. New Phytol. 2008, 177, 706–714.
- Stefanowicz, A.M.; Majewska, M.L.; Stanek, M.; Nobis, M.; Zubek, S. Differential influence of four invasive plant species on soil physicochemical properties in a pot experiment. J. Soils Sediments 2018, 18, 1409–1423.
- Van, K.M.; Weber, E.; Fischer, M. A meta-analysis of trait differences between invasive and non-invasive plant species. Ecol. Lett. 2010, 13, 235–245.
- Beck, M.W.; Alahuhta, J. Ecological determinants of Potamogeton taxa in glacial lakes: Assemblage composition, species richness, and species-level approach. Aquat. Sci. 2017, 79, 427–441.
- Dawson, W.; Fischer, M.; Kleunen, M.V. Common and rare plant species respond differently to fertilisation and competition, whether they are alien or native. Ecol. Lett. 2012, 15, 873–880.
- Sterner, R.W.; Elser, J.J. Ecological Stoichiometry: The Biology of Elements from Molecules to the Biosphere; Princeton University Press: Princeton, NJ, USA, 2002; pp. 225–226.
- Martina, J.P.; Currie, W.S.; Goldberg, D.E.; Elgersma, K.J. Nitrogen loading leads to increased carbon accretion in both invaded and uninvaded coastal wetlands. Ecosphere 2016, 7, e01459.
- Daniel, S.; Betsy, V.H. Positive Interactions of Nonindigenous Species: Invasional Meltdown? Biol. Invasions 1999, 1, 21–32.
- Yessoufou, K.; Bezeng, B.S.; Gaoue, O.G.; Bengu, T.; van der Bank, M. Phylogenetically diverse native systems are more resistant to invasive plant species on Robben Island, South Africa. Genome 2019, 62, 217–228.
- Sun, S.; Chen, J.; Feng, W.; Zhang, C.; Huang, K.; Guan, M.; Sun, J.; Liu, M.; Feng, Y. Plant strategies for nitrogen acquisition and their effects on exotic plant invasions. Biodivers. Sci. 2021, 29, 72–80.
- Güsewell, S.; Bollens, U. Composition of plant species mixtures grown at various N:P ratios and levels of nutrient supply. Basic Appl. Ecol. 2003, 4, 453–466.
- Müller, I.; Schmid, B.; Weiner, J. The effect of nutrient availability on biomass allocation patterns in 27 species of herbaceous plants. Perspect. Plant Ecol. Evol. Syst. 2000, 3, 115–127.
- Stark, J.M.; Norton, J.M. The invasive annual cheatgrass increases nitrogen availability in 24-year-old replicated field plots. Oecologia 2015, 177, 799–809.
- Kuebbing, S.E.; Classen, A.T.; Simberloff, D. Two co-occurring invasive woody shrubs alter soil properties and promote subdominant invasive species. J. Appl. Ecol. 2014, 51, 124–133.
- Lee, M.R.; Flory, S.L.; Phillips, R.P. Positive feedbacks to growth of an invasive grass through alteration of nitrogen cycling. Oecologia 2012, 170, 457–465.
- Elgersma, K.J.; Ehrenfeld, J.G.; Yu, S.; Vor, T. Legacy effects overwhelm the short-term effects of exotic plant invasion and restoration on soil microbial community structure, enzyme activities, and nitrogen cycling. Oecologia 2011, 167, 733.
- Dassonville, N.; Vanderhoeven, S.; Vanparys, V.; Hayez, M.; Gruber, W.; Meerts, P. Impacts of Alien Invasive Plants on Soil Nutrients Are Correlated with Initial Site Conditions in NW Europe. Oecologia 2008, 157, 131–140.
- Li, W.H.; Zhang, C.B.; Jiang, H.B.; Xin, G.R.; Yang, Z.Y. Changes in Soil Microbial Community Associated with Invasion of the Exotic Weed, Mikania micrantha H.B.K. Plant Soil 2006, 281, 309–324.
- Ulm, F.; Jacinto, J.; Cruz, C.; Maguas, C. How to Outgrow Your Native Neighbour? Belowground Changes under Native Shrubs at an Early Stage of Invasion. Land Degrad. Dev. 2017, 28, 2380–2388.
- Li, J.; Lu, H.; Liu, J.; Hong, H.; Yan, C. The influence of flavonoid amendment on the absorption of cadmium in Avicennia marina roots. Ecotoxicol. Environ. Saf. 2015, 120, 1–6.
- Li, J.; Liu, J.; Lu, H.; Jia, H.; Yu, J.; Hong, H.; Yan, C. Influence of the phenols on the biogeochemical behavior of cadmium in the mangrove sediment. Chemosphere 2016, 144, 2206–2213.
- Uddin, M.N.; Robinson, R.W. Can nutrient enrichment influence the invasion of Phragmites australis? Sci. Total Environ. 2018, 613, 1449–1459.
- Uddin, M.D.N.; Robinson, R.W. Responses of plant species diversity and soil physical-chemicalmicrobial properties to Phragmites australis invasion along a density gradient. Sci. Rep. 2017, 7, 11007.
- Dalle Fratte, M.; Bolpagni, R.; Brusa, G.; Caccianiga, M.; Pierce, S.; Zanzottera, M.; Cerabolini, B.E. Alien plant species invade by occupying similar functional spaces to native species. Flora 2019, 257, 151419.
- Shannon-Firestone, S.; Reynolds, H.L.; Phillips, R.P.; Flory, S.L.; Yannarell, A. The role of ammonium oxidizing communities in mediating effects of an invasive plant on soil nitrification. Soil Biol. Biochem. 2015, 90, 266–274.
- Rossiter-Rachor, N.A.; Setterfield, S.A.; Douglas, M.M.; Hutley, L.B.; Cook, G.D.; Schmidt, S. Invasive Andropogon gayanus (gamba grass) is an ecosystem transformer of nitrogen relations in Australian savanna. Ecol. Appl. 2009, 19, 1546–1560.
- Davis, M.A.; Grime, J.P.; Thompson, K. Fluctuating resources in plant communities: A general theory of invasibility. J. Ecol. 2000, 88, 528–534.
- Wan, L.-Y.; Qi, S.-S.; Dai, Z.-C.; Zou, C.B.; Song, Y.-G.; Hu, Z.-Y.; Zhu, B.; Du, D.-L. Growth responses of Canada goldenrod (Solidago canadensis L.) to increased nitrogen supply correlate with bioavailability of insoluble phosphorus source. Ecol. Res. 2018, 33, 261–269.
- Mohren, G.M.J.; Burger, F.W. Phosphorus deficiency induced by nitrogen input in Douglas fir in the Netherlands. Plant Soil 1986, 95, 191–200.
- Gress, S.E.; Nichols, T.D.; Northcraft, C.C.; Peterjohn, W.T. Nutrient Limitation in Soils Exhibiting Differing Nitrogen Availabilities: What Lies beyond Nitrogen Saturation? Ecology 2007, 88, 119–130.
- Zhang, R.; Zhou, Z.C.; Luo, W.J.; Wang, Y.; Feng, Z.P. Effects of nitrogen deposition on growth and phosphate efficiency of Schima superba of different provenances grown in phosphorus-barren soil. Plant Soil 2013, 370, 435–445.
- Wan, L.-Y.; Qi, S.-S.; Zou, C.B.; Dai, Z.-C.; Zhu, B.; Song, Y.-G.; Du, D.-L. Phosphorus addition reduces the competitive ability of the invasive weed Solidago canadensis under high nitrogen conditions. Flora 2018, 240, 68–75.
- Harpole, W.S. Resource-Ratio Theory and the Control of Invasive Plants. Plant Soil 2006, 280, 23–27.
- Seabloom, E.W.; Harpole, W.S.; Reichman, O.J.; Tilman, D. Invasion, competitive dominance, and resource use by exotic and native California grassland species. Proc. Natl. Acad. Sci. USA 2003, 100, 13384–13389.
More
