Non-muscle-invasive bladder cancer (NMIBC) is characterized by a high rate of cure, but also by a non-negligible probability of recurrence and risk progression to muscle-invasive disease. NMIBC management requires a proper local resection and staging, followed by a risk-based treatment with intravesical agents. For many years, the current gold standard treatment for patients with intermediate or high-risk disease is transurethral resection of the bladder (TURB) followed by intravesical bacillus Calmette–Guérin (BCG) instillations. Unfortunately, in about half of high-risk patients, intravesical BCG treatment fails and NMIBC persists or recurs early. While radical cystectomy remains the gold standard for these patients, new therapeutic targets are being individuated and studied. Radical cystectomy in fact can provide an excellent long-term disease control, but can deeply interfere with quality of life. In particular, the enhanced immune checkpoints expression shown in BCG-unresponsive patients and the activity of immune checkpoints inhibitors (ICIs) in advanced bladder cancer provided the rationale for testing ICIs in NMIBC. Recently, pembrolizumab has shown promising activity in BCG-unresponsive NMIBC patients, obtaining FDA approval. Meanwhile multiple novel drugs with alternative mechanisms of action have proven to be safe and effective in NMIBC treatment and others are under investigation.
- non-muscle-invasive bladder cancer
- BGC-unresponsive
- immunotherapy
- immune-checkpoint inhibitors
- pembrolizumab
1. Immunotherapy in NMIBC: From BCG to the New Horizons of ICIs
1.1. BCG Administration Drives an Antitumour Innate and Adaptative Immune Response in NMIBC
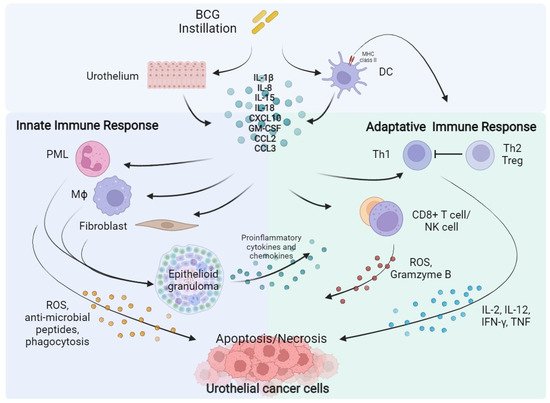
1.2. PD-L1 and PD-1 Expression Is Associated to BCG Immune-Resistance
1.3. ICIs for the Treatment of Advanced Urothelial Cancer
1.4. ICIs Activity in BCG-Unresponsive NMIBC
| Agent/ Target |
NCT/ Acronym |
Phase | Primary Endpoint | Patients Enrolled | Median Follow Up | Results |
|---|---|---|---|---|---|---|
| Pembrolizumab * ICI Anti-PD1 IgG4/kappa |
NCT02625961 KEYNOTE-057 [28] |
II | CRR of high-risk NMIBC | Cohort A (CIS): 101 pts Cohort B (Non-CIS): 47 pts |
36.4 mos. | Cohort A: 41% (39 out of 96 pts, 95% CI 30.7–51.1%) |
| Atezolizumab |
| NCT/Acronym | Status | Phase | Drug(s) | Control | Primary Endpoints |
|---|
| NCT/Acronym | Status | Phase | Drug(s) | Target or Mechanism | Primary Endpoints | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICI | ||||||||||||
Anti-PD-L1 IgG1 |
NCT02844816 | |||||||||||
| (a) BCG-unresponsive or BCG-intolerant NMIBC | SWOG S1605 [29] |
II | CRR at 25 weeks in CIS-cohort | |||||||||
| (a) BCG-unresponsive or BCG-intolerant NMIBC | ||||||||||||
| NCT05014139 | CIS cohort: 70 pts pre-planned Non-CIS cohort: 65 pts pre-planned | |||||||||||
| NCT05120622 Rideau |
Recruiting | 1, 2 | Durvalumab, tremelimumab | Not yet recruiting— | NR | CIS cohort: 27% (20 out of 74 pts, 95% CI NR) | ||||||
| TRAEs, MTD | Nadofaragene firadenovec rAd-IFNa2b/Syn3 |
NCT02773849 [30] | III | CRR at 12 mos. in CIS-cohort | CIS-cohort: 107 pts Non-CIS cohort: 50 pts |
19.7 mos. | CIS-cohort: 53.4% (55 out of 103 patients, 95% CI 43.3–63.3%) | |||||
| NCT04738630 | Recruiting | 2 | HX008 (Pucotenlimab) | — | CRR, EFS | Oportuzumab Monatox EpCAM scFv linked to ETA |
NCT02449239 [31] | III | CRR in CIS-cohort | 126 pts CIS-cohort: 89 pts |
NR | CIS-cohort: 40% (95% CI NR) |
| 1 |
| Enfortumab Vedotin | |||||||||||
| ADC against Nectin-4 | TRAEs, DLT | ||||||||||
| NCT04917809 | Not yet recruiting | 2 | Erdafitinib | FGFR-TKI | ORR | NCT04706598 | |||||
| NCT04799847Recruiting | Not yet recruiting1, 2 | 1, 2Camrelizumab | Catumaxomab— | Bispecific (anti-EpCAM, anti-CD3) AbMTD, RFS | |||||||
| DLT, TRAEs | NCT04640623 SunRISe-1 | Recruiting | 2 | TAR-200, Cetrelimab | TAR-200 or Cetrelimab | CRR | |||||
| NCT04498702 | Completed | 2 | APL-1202 | MetAP2 inhibitor | RFR | NCT04387461 CORE-001 |
Recruiting | 2 | CG0070, Pembrolizumab | — | CRR |
| NCT04452591 BOND-003 |
Recruiting | 3 | CG0070 | Oncolytic adenovirus | CRR | NCT04164082 | Recruiting | 2 | Pembrolizumab, gemcitabine | — | CRR in CIS subpopulation, EFS |
| NCT04172675 | Recruiting | 2 | Erdafitinib vs. gemcitabine/MMC | FGFR-TKI | RFS | NCT03950362 PREVERT | Not yet recruiting | 2 | Avelumab, RDT | — | RFS |
| NCT03914794 | Recruiting | 2 | Pemigatinib | FGFR1-3-TKI | CRR | NCT03759496 | |||||
| NCT03022825 Recruiting |
2 | Durvalumab | — | MTD, RFS | |||||||
| QUILT-3.032 | Recruiting | 2, 3 | BCG, ALT-803 | IL-15 superagonist | CRR, DFR | NCT03519256 CheckMate 9UT |
Active, not recruiting | 2 | Nivolumab, BMS-986205 (Linrodostat mesylate) | Nivolumab | CRR, DoR |
| NCT03317158 ADAPT-BLADDER |
Recruiting | 1, 2 | |||||||||
| NCT02009332 | Completed | 1, 2 | Nab-sirolimus, gemcitabine | mTOR inhibitor | DLT, CRR | Durvalumab, RDT | — | RP2D, RFS | |||
| NCT01731652 | Completed | 2 | Vesimune | TLR-7 agonist | CRR | NCT04149574 CheckMate 7G8 |
Recruiting | 3 | Nivolumab, BCG | ||
| NCT02371447 | Active, not recruiting | 1, 2BCG | VPM1002BCEFS | ||||||||
| Modified BCG | DLT, RFR | NCT04106115 DURANCE |
Not yet recruiting | 1, 2 | Durvalumab, S-488210/S-488211 vaccine | — | DLT, DFSR | ||||
| NCT03892642 | |||||||||||
| (b) BCG-naïve NMIBC | ABC Trial | Active, not recruiting | 1, 2 | Avelumab, BCG | — | DLT | |||||
| NCT04736394 ASCERTAIN |
Not yet recruiting | (b) BCG-naïve NMIBC | |||||||||
| 3 | APL-1202 vs. epirubicin | MetAP2 inhibitor | NCT04922047 TACBIN-01 |
Recruiting | 1, 2 | Tislelizumab, BCG | — | DLT | |||
| EFS | |||||||||||
| NCT02138734 | Recruiting | 1, 2 | ALT-803, BCG | IL-15 superagonist | CRR, DFS | NCT04730232 | Recruiting | 2 | Tislelizumab, nab-paclitaxel | — | CRR |
| NCT04165317 * CREST |
Recruiting | 3 | Sasanlimab, BCG | BCG | EFS, CRR | ||||||
| NCT03799835 ALBAN |
Recruiting | 3 | Atezolizumab, 1y BCG | BCG | RFS | ||||||
| NCT03711032 * KEYNOTE-676 |
Recruiting | 3 | Pembrolizumab, BCG | BCG | CRR, EFS | ||||||
| NCT03528694 POTOMAC |
Active, not recruiting | 3 | Durvalumab, BCG | BCG | DFS | ||||||
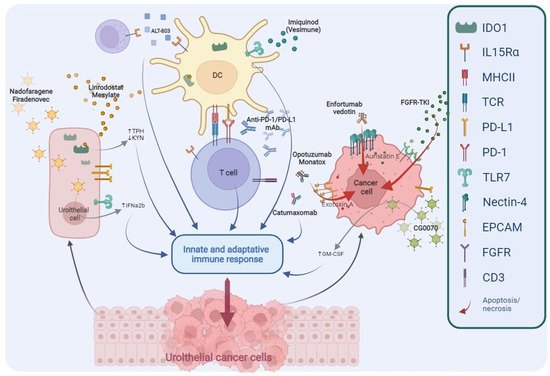
2. Alternative Targets: The Way to Develop New Effective Drugs
References
- Morales, A.; Eidinger, D.; Bruce, A.W. Intracavitary Bacillus Calmette-Guerin in the treatment of superficial bladder tumors. J. Urol. 1976, 116, 180–182.
- Larsen, E.S.; Joensen, U.N.; Poulsen, A.M.; Goletti, D.; Johansen, I.S. Bacillus Calmette–Guérin immunotherapy for bladder cancer: A review of immunological aspects, clinical effects and BCG infections. Apmis 2020, 128, 92–103.
- Ingersoll, M.A.; Albert, M.L. From infection to immunotherapy: Host immune responses to bacteria at the bladder mucosa. Mucosal Immunol. 2013, 6, 1041–1053.
- Teppema, J.S.; de Boer, E.C.; Steerenberg, P.A.; van der Meijden, A.P. Morphological aspects of the interaction of Bacillus Calmette-Guérin with urothelial bladder cells in vivo and in vitro: Relevance for antitumor activity? Urol. Res. 1992, 20, 219–228.
- Bisiaux, A.; Thiounn, N.; Timsit, M.-O.; Eladaoui, A.; Chang, H.-H.; Mapes, J.; Mogenet, A.; Bresson, J.-L.; Prié, D.; Béchet, S.; et al. Molecular analyte profiling of the early events and tissue conditioning following intravesical bacillus calmette-guerin therapy in patients with superficial bladder cancer. J. Urol. 2009, 181, 1571–1580.
- Ludwig, A.T.; Moore, J.M.; Luo, Y.; Chen, X.; Saltsgaver, N.A.; O’Donnell, M.A.; Griffith, T.S. Tumor necrosis factor-related apoptosis-inducing ligand: A novel mechanism for Bacillus Calmette-Guérin-induced antitumor activity. Cancer Res. 2004, 64, 3386–3390.
- Lage, J.M.; Bauer, W.C.; Kelley, D.R.; Ratliff, T.L.; Catalona, W.J. Histological parameters and pitfalls in the interpretation of bladder biopsies in bacillus Calmette-Guerin treatment of superficial bladder cancer. J. Urol. 1986, 135, 916–919.
- Mitropoulos, D.N. Novel insights into the mechanism of action of intravesical immunomodulators. In Vivo 2005, 19, 611–621.
- Stefanini, G.F.; Bercovich, E.; Mazzeo, V.; Grigioni, W.F.; Emili, E.; D’Errico, A.; Lo Cigno, M.; Tamagnini, N.; Mazzetti, M. Class I and class II HLA antigen expression by transitional cell carcinoma of the bladder: Correlation with T-cell infiltration and BCG treatment. J. Urol. 1989, 141, 1449–1453.
- Ikeda, N.; Toida, I.; Iwasaki, A.; Kawai, K.; Akaza, H. Surface antigen expression on bladder tumor cells induced by bacillus Calmette-Guérin (BCG): A role of BCG internalization into tumor cells. Int. J. Urol. 2002, 9, 29–35.
- Luo, Y. Blocking IL-10 enhances bacillus Calmette-Guérin induced T helper Type 1 immune responses and anti-bladder cancer immunity. Oncoimmunology 2012, 1, 1183–1185.
- Pettenati, C.; Ingersoll, M.A. Mechanisms of BCG immunotherapy and its outlook for bladder cancer. Nat. Rev. Urol. 2018, 15, 615–625.
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264.
- Topalian, S.L.; Taube, J.M.; Anders, R.A.; Pardoll, D.M. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat. Rev. Cancer 2016, 16, 275–287.
- Kates, M.; Matoso, A.; Choi, W.; Baras, A.S.; Daniels, M.J.; Lombardo, K.; Brant, A.; Mikkilineni, N.; McConkey, D.J.; Kamat, A.M.; et al. Adaptive immune resistance to intravesical BCG in non–muscle invasive bladder cancer: Implications for prospective BCG-unresponsive trials. Clin. Cancer Res. 2020, 26, 882–891.
- Pierconti, F.; Raspollini, M.R.; Martini, M.; Larocca, L.M.; Bassi, P.F.; Bientinesi, R.; Baroni, G.; Minervini, A.; Petracco, G.; Pini, G.M.; et al. PD-L1 expression in bladder primary in situ urothelial carcinoma: Evaluation in BCG-unresponsive patients and BCG responders. Virchows Arch. 2020, 477, 269–277.
- Hashizume, A.; Umemoto, S.; Yokose, T.; Nakamura, Y.; Yoshihara, M.; Shoji, K.; Wada, S.; Miyagi, Y.; Kishida, T.; Sasada, T. Enhanced expression of PD-L1 in non-muscle-invasive bladder cancer after treatment with Bacillus Calmette-Guerin. Oncotarget 2018, 9, 34066–34078.
- Fukumoto, K.; Kikuchi, E.; Mikami, S.; Hayakawa, N.; Matsumoto, K.; Niwa, N.; Oya, M. Clinical Role of Programmed Cell Death-1 Expression in Patients with Non-muscle-invasive Bladder Cancer Recurring After Initial Bacillus Calmette–Guérin Therapy. Ann. Surg. Oncol. 2018, 25, 2484–2491.
- Chevalier, M.F.; Schneider, A.K.; Cesson, V.; Dartiguenave, F.; Lucca, I.; Jichlinski, P.; Nardelli-Haefliger, D.; Derré, L. Conventional and PD-L1-expressing Regulatory T Cells are Enriched During BCG Therapy and may Limit its Efficacy. Eur. Urol. 2018, 74, 540–544.
- Copland, A.; Sparrow, A.; Hart, P.; Diogo, G.R.; Paul, M.; Azuma, M.; Reljic, R. Bacillus Calmette-Guérin Induces PD-L1 Expression on Antigen-Presenting Cells via Autocrine and Paracrine Interleukin-STAT3 Circuits. Sci. Rep. 2019, 9, 3655.
- Alexandrov, L.B.; Nik-Zainal, S.; Wedge, D.C.; Aparicio, S.A.J.R.; Behjati, S.; Biankin, A.V.; Bignell, G.R.; Bolli, N.; Borg, A.; Børresen-Dale, A.L.; et al. Signatures of mutational processes in human cancer. Nature 2013, 500, 415–421.
- Samstein, R.M.; Lee, C.-H.; Shoushtari, A.N.; Hellmann, M.D.; Shen, R.; Janjigian, Y.Y.; Barron, D.A.; Zehir, A.; Jordan, E.J.; Omuro, A.; et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat. Genet. 2019, 51, 202–206.
- Bellmunt, J.; de Wit, R.; Vaughn, D.J.; Fradet, Y.; Lee, J.-L.; Fong, L.; Vogelzang, N.J.; Climent, M.A.; Petrylak, D.P.; Choueiri, T.K.; et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N. Engl. J. Med. 2017, 376, 1015–1026.
- Roviello, G.; Catalano, M.; Santi, R.; Palmieri, V.E.; Vannini, G.; Galli, I.C.; Buttitta, E.; Villari, D.; Rossi, V.; Nesi, G. Immune checkpoint inhibitors in urothelial bladder cancer: State of the art and future perspectives. Cancers 2021, 13, 4411.
- Powles, T.; Park, S.H.; Voog, E.; Caserta, C.; Valderrama, B.P.; Gurney, H.; Kalofonos, H.; Radulović, S.; Demey, W.; Ullén, A.; et al. Avelumab Maintenance Therapy for Advanced or Metastatic Urothelial Carcinoma. N. Engl. J. Med. 2020, 383, 1218–1230.
- Bajorin, D.F.; Witjes, J.A.; Gschwend, J.E.; Schenker, M.; Valderrama, B.P.; Tomita, Y.; Bamias, A.; Lebret, T.; Shariat, S.F.; Park, S.H.; et al. Adjuvant Nivolumab versus Placebo in Muscle-Invasive Urothelial Carcinoma. N. Engl. J. Med. 2021, 384, 2102–2114.
- Rosenberg, J.E.; Hoffman-Censits, J.; Powles, T.; van der Heijden, M.S.; Balar, A.V.; Necchi, A.; Dawson, N.; O’Donnell, P.H.; Balmanoukian, A.; Loriot, Y.; et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: A single-arm, multicentre, phase 2 trial. Lancet 2016, 387, 1909–1920.
- Balar, A.V.; Kamat, A.M.; Kulkarni, G.S.; Uchio, E.M.; Boormans, J.L.; Roumiguié, M.; Krieger, L.E.M.; Singer, E.A.; Bajorin, D.F.; Grivas, P.; et al. Pembrolizumab monotherapy for the treatment of high-risk non-muscle-invasive bladder cancer unresponsive to BCG (KEYNOTE-057): An open-label, single-arm, multicentre, phase 2 study. Lancet Oncol. 2021, 22, 919–930.
- Black, P.C.; Tangen, C.; Singh, P.; McConkey, D.J.; Lucia, S.; Lowrance, W.T.; Koshkin, V.S.; Stratton, K.L.; Bivalacqua, T.; Kassouf, W.; et al. Phase II trial of atezolizumab in BCG-unresponsive non-muscle invasive bladder cancer: SWOG S1605 (NCT #02844816). J. Clin. Oncol. 2021, 39, 4541.
- Boorjian, S.A.; Alemozaffar, M.; Konety, B.R.; Shore, N.D.; Gomella, L.G.; Kamat, A.M.; Bivalacqua, T.J.; Montgomery, J.S.; Lerner, S.P.; Busby, J.E.; et al. Intravesical nadofaragene firadenovec gene therapy for BCG-unresponsive non-muscle-invasive bladder cancer: A single-arm, open-label, repeat-dose clinical trial. Lancet Oncol. 2021, 22, 107–117.
- Shore, N.; O’Donnell, M.; Keane, T.; Jewett, M.A.; Kulkarni, G.S.; Dickstein, R.; Wolk, F.; Dunshee, C.; Belkoff, L.; Dillon, R.L.; et al. PD03-02 Phase 3 results of Vicineum in BCG-unresponsive Non-Muscle Invasive Bladder Cancer. J. Urol. 2020, 203, e72.
- Black, P.C.; Tangen, C.; Singh, P.; McConkey, D.J.; Lucia, S.; Lowrance, W.T.; Koshkin, V.S.; Stratton, K.L.; Bivalacqua, T.; Sharon, E.; et al. Phase II trial of atezolizumab in BCG-unresponsive non-muscle invasive bladder cancer: SWOG S1605 (NCT #02844816). J. Clin. Oncol. 2020, 38, 5022.
- Michael, C.; Abhishek, T.; Sanjay, P.; Daniel, Z.; Yuejin, W.; Riza, F.; Kelly, S. LBA02-04 Novel weekly immunotherapy dosing with avelumab tolerated during Bacillus Calmette-Guerin induction therapy: Initial results of the ABC trial. J. Urol. 2021, 206, e1177.
- Definition of Multipeptide Vaccine S-588210—NCI Drug Dictionary—National Cancer Institute. Available online: https://www.cancer.gov/publications/dictionaries/cancer-drug/def/multipeptide-vaccine-s-588210 (accessed on 23 November 2021).
