Gestational diabetes mellitus (GDM) is characterized by a set of metabolic complications arising from adaptive failures to the pregnancy period. Estimates point to a prevalence of 3 to 15% of pregnancies. Its etiology includes intrinsic and extrinsic aspects the progenitress, which may contribute to the pathophysiogenesis of GDM. Recently, researchers have identified that the intestinal microbiota participates in the development of the disease, both through its influence on insulin resistance, as well as on pro-oxidant and pro-inflammatory products, which are potentially harmful to the health of the maternal-fetal binomial, in the short and long term.In this context, our objective was to gather evidence on the modulation of the intestinal microbiota, through the use of probiotics and prebiotics, with antioxidant and anti-inflammatory properties, which can mitigate the endogenous processes of GDM, favoring the health of the mother and her children and , in a future perspective, to alleviate this critical public health problem.
- pregnancy
- dysbiosis
- Gestational diabetes mellitus
1. Gestational Diabetes Mellitus
GDM reflects a set of endocrine complications arising from adaptive organ failure, considered the most common metabolic disorder of the pregnancy period [1]. Its recognition occurs through the identification of spontaneous hyperglycemia, during pregnancy, and without precedents [1][2]. Global estimates indicate that gestational hyperglycemia affects an average of 16.2% of pregnancies, among which 86.4% are due to GDM (Box 1) [3].| Global Prevalence | Prevalence by Region | ||
| Criteria | Time Course | Fasting Glucose (mg/dL) | Glucose Overload | Oral Glucose Tolerance Test (mg/dL) | ||
| Hyperglycemia in pregnancy | 16.2% | Africa | 10.4% | |||
| 1 h | 2 h | 3 h | Western Pacific | 12.6% | ||
1.2. Etiology
| Risk Factor | Mechanism of Action | Reference | |||||||
| Advanced chronological age (>35 years old) |
|
[23][24] | |||||||
| O’Sullivan & Mahan (1964) [5] | Detected at any time during pregnancy | ||||||||
| Family history |
| 90 |
|
[25]100 g of glucose | [165 | 145 | 26125 | ] | South America and Central America |
| O’Sullivan & Mahan (1964) [5] | 13.1% | ||||||||
| adapted by National Diabetes Data Group (NDDG) (1979) [6] | 105 | 100 g of glucose | 190 | 165 | 145 | ||||
| Genetic factors |
|
[25][26][27] | North America and Caribbean | 14.6% | |||||
| Carpenter & Coustan (1982) [7] |
95 | ||||||||
|
| 100 g of glucose |
| 180 |
| 155 | 140 |
|
Europe | |
| World Health Organization (WHO) (1999) [ | 16.2% | ||||||||
| Middle East and North Africa | 21.8% | ||||||||
| South East Asia | 24.2% | ||||||||
| Source: Adapted from International Diabetes Federation [3]. | |||||||||
1.1. Screening and Diagnosis
| [25][27][28] | |||||||||
| 10] | |||||||||
| Race/ | 126 | Ethnicity |
| 75 g of Glucose |
Not measured |
[29140 | ][30 | Not measured |
|
| ] | International Association of Diabetes in Pregnancy Study Group (IADPSG (2010) [11] | 24–28 gestational weeks |
92 | 75 g of Glucose |
180 | 153 |
| Geographic features | ||
| ||
|
[12][16] | |
| Socio-economic |
|
[30][31] |
| Overweight |
|
[20][32] |
|
[33][34] | |
| Westernized diet |
|
[22][35] |
| Sedentary lifestyle |
|
[36][37] |
| Exposure to chemicals |
|
[38][39][40][41][42][43] |
|
[44][45][46] | |
| Polycystic Ovary Syndrome (POS) |
|
[47][48] |
| Vitamin D Deficiency |
|
[49][50] |
| Adverse birth conditions of the mother (Fetal program) |
|
[51][52][53][54][55] |
1.3. Maternal and Perinatal Outcomes in the Gestational Diabetes Mellitus
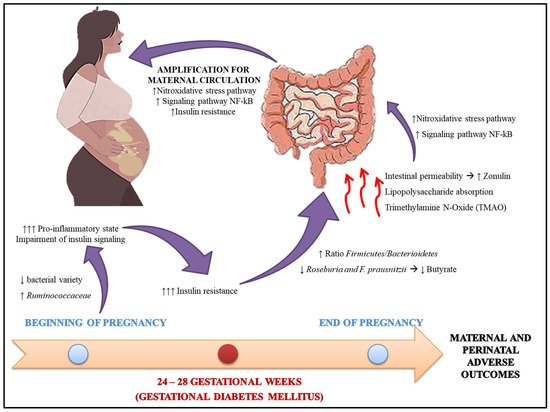
2. Intestinal Microbiota and GDM
| Source Sample | Population | Size * | Supplementation | Dose/Duration | Main Findings |
|---|---|---|---|---|---|
| Karamali et al. (2016) [94] | Iran | I: 30 C: 30 |
L. acidophilus + L. casei + B. bifidum | 2 × 109 CFU/ 6 weeks |
Supplementation with probiotics ↓FBG, serum insulin, TG, and VLDL-c, and improved insulin resistance indexes. |
| Hajifaraji et al. (2018) [91] | Iran | I: 27 C: 29 |
L. acidophilus LA-5 + B. BB-12 + S. thermophilus STY-31 + L. delbrueckii bulgaricus + LBY-27 | >4 × 109 CFU/ 8 weeks |
Supplementation with probiotics significantly ↓CRP and TNF-α. MDA, GPx and GR in women in the intervention group. |
| Kijmanawat et al. (2019) [95] | Thailand | I: 28 C: 29 |
Bifidobacterium + Lactobacillus | 2 × 109 CFU/ 4 weeks |
In women with diet-controlled GDM, supplementation with probiotics ↓FBG and insulin resistance compared with the control. |
| Babadi et al. (2018) [96] | Iran | I: 24 C: 24 |
L. casei + B. bifidum + L. fermentum + L. acidophilus | 2 × 109 CFU/ 6 weeks |
Probiotic supplementation improved the expression of genes related to insulin; glycemic control; inflammation; lipid profile, and oxidative stress markers, such as ↓MDA and ↑TAC, compared with the control. |
| Badehnoosh et al. (2018) [97] | Iran | I: 30 C: 30 |
L. acidophilus + L. casei + B. bifidum | 2 × 109 CFU/ 6 weeks |
Probiotic supplementation improved FBG, and CRP, ↑TAC, and ↓MDA, without affecting pregnancy outcomes. |
| Nabhani et al. (2018) [98] | Iran | I: 45 C: 45 |
L. acidophilus + L. plantarum + L. fermentum + L. gasseri + FOS | 1.5–7.0 × 109–10 CFU + 38.5 mg/ 6 weeks |
Symbiotics had no effect on FBG and insulin resistance/sensitivity indexes. However, an ↑ in HDL-c and TAC was seen, and a ↓ was seen in blood pressure in the intervention group. |
| Jamilian et al. (2019) [99] | Iran | I: 29 C: 28 |
L. acidophilus + B. bifidum + L. reuteri + L. fermentum + Vitamin D | 8 × 109 CFU/ 6 weeks +50.000 UI every 2 weeks |
↓FBG, serum insulin, CRP, and MDA; ↑TAC and GSH; and improved insulin resistance scores. |
| Karamali et al. (2018) [100] | Iran | I: 30 C: 30 |
L. acidophilus + L. casei + B. bifidum + Inulin | 2 × 109 CFU/ 6 weeks +800 mg |
Symbiotic supplementation ↓CRP and MDA; ↑TAC and GSH; and↓ the rates of cesarean section, hyperbilirubinemia and hospitalization in NB, without affecting other pregnancy outcomes. |
| Ahmadi et al. (2016) [101] | Iran | I: 35 C: 35 |
L. acidophilus + L. casei + B. bifidum + inulin | 2 × 109 CFU/ 6 weeks +800 mg |
Symbiotics ↑ insulin metabolism markers, and the insulin sensitivity index as well as ↓VLDL-c and TG. |
| Jafarnejad et al. (2016) [102] | Iran | I: 41 C: 41 |
S. thermophilus + B. breve + B. longum + B. infantis + L. acidophilus + L. plantarum + L. paracasei + L. delbrueckii subsp. Bulgaricus | 15 × 109 CFU/ 8 weeks |
No differences were observed in FBG, glycated hemoglobin, serum insulin, and insulin resistance indices. However, ↓CRP, IL-6, and TNF-α were observed, without changes in IL-10 and IFN-γ. |
| Dolatkhah et al. (2015) [103] | Turkey | I: 29 C: 27 |
L. acidophilus LA-5 + B. BB-12 + Streptococcus thermophilus + STY-31 + L. delbrueckii bulgaricus LBY-27 | >4 × 109 CFU/ 8 weeks |
↓FBG and insulin resistance index, and less weight gain in those in the intervention group. |
| Lindsay et al. (2015) [104] | Ireland | I: 74 C: 75 |
L. salivarius | 1 × 109 CFU/ 6 weeks |
No beneficial effect on glycemic control or pregnancy outcomes. ↓ in total and LDL-c in the supplemented group. |
References
- American Diabetes Association. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2018. Diabetes Care 2018, 41 (Suppl. 1), S13–S27.
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2021. Diabetes Care 2021, 44 (Suppl. 1), S15–S33.
- International Diabetes Federation. IDF Diabetes Atlas, 8th ed.; International Diabetes Federation: Brussels, Belgium, 2017; ISBN 978-2-930229-87-4.
- Organização Pan-Americana de Saúde; Ministério da Saúde; Federação Brasileira das Associações de Ginecologia e Obstetrícia; Sociedade Brasileira de Diabetes. Rastreamento e Diagnóstico de Diabetes Mellitus Gestacional no Brasil—Brasília. 2017. E-book, pp. 15–19. Available online: https://www.febrasgo.org.br/images/pec/CNE_pdfs/Rastreamento-Diabetes.pdf (accessed on 15 October 2021).
- O’Sullivan, J.B.; Mahan, C.M. Criteria for the oral glucose tolerance test in pregnancy. Diabetes 1964, 13, 278–285.
- National Diabetes Data Group. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. National Diabetes Data Group. Diabetes 1979, 28, 1039–1057.
- Carpenter, M.W.; Coustan, D.R. Criteria for screening tests for gestational diabetes. Am. J. Obstet. Gynecol. 1982, 144, 768–773.
- American College of Obstetrics and Gynecology. Proceedings of the Third International Workshop-Conference on Gestational Diabetes Mellitus. November 8–10,1990, Chicago, Illinois. Diabetes 1991, 40 (Suppl. 2), 1–201.
- HAPO Study Cooperative Research Group. Hyperglycemia and adverse pregnancy outcomes. N. Engl. J. Med. 2008, 358, 1991–2002.
- World Health Organization. Definition, Diagnosis and Classification of Diabetes Mellitus and its Complications; World Health Organization: Geneva, Switzerland, 1999.
- International Association of Diabetes and Pregnancy Study Groups Consensus Panel. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010, 33, 676–682.
- Organização Pan-Americana da Saúde. Rastreamento e Diagnóstico de Diabetes Mellitus Gestacional No Brasil. Ministério da Saúde. Federação Brasileira das Associações de Ginecologia e Obstetrícia. (DF): OPAS; Sociedade Brasileira de Diabetes: São Paulo, Brazil, 2016.
- HAPO Study Cooperative Research Group. The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study. Int. J. Gynaecol. Obstet. 2002, 78, 69–77.
- Blumer, I.; Hadar, E.; Hadden, D.R.; Jovanovic, L.; Mestman, J.H.; Murad, M.H.; Yogev, Y. Diabetes and pregnancy: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2013, 98, 4227–4249.
- World Health Organization. Diagnostic Criteria and Classification of Hyperglycaemia First Detected in Pregnancy: A World Health Organization Guideline; WHO Press: Geneva, Switzerland, 2013; Available online: http://apps.who.int/iris/bitstream/10665/85975/1/WHO_NMH_MND_13.2_eng.pdf (accessed on 15 October 2021).
- Hod, M.; Kapur, A.; Sacks, D.A.; Hadar, E.; Agarwal, M.; Di Renzo, G.C.; Roura, L.C.; McIntyre, H.D.; Morris, J.L.; Divakar, H. The International Federation of Gynecology and Obstetrics (FIGO) initiative on gestational diabetes mellitus: A pragmatic guide for diagnosis, management, and care. Int. J. Gynaecol. Obstet. 2015, 131 (Suppl. 3), S173–S211.
- McIntyre, H.D.; Sacks, D.A.; Barbour, L.A.; Feig, D.S.; Catalano, P.M.; Damm, P.; McElduff, A. Issues with the diagnosis and classification of hyperglycemia in early pregnancy. Diabetes Care 2016, 39, 53–54.
- Schmidt, M.I.; Duncan, B.B.; Reichelt, A.J.; Branchtein, L.; Matos, M.C.; e Forti, A.C.; Spichler, E.R.; Pousada, J.M.; Teixeira, M.M.; Yamashita, T.; et al. Gestational diabetes mellitus diagnosed with a 2-h 75-g oral glucose tolerance test and adverse pregnancy outcomes. Diabetes Care 2001, 24, 1151–1155.
- Trujillo, J.; Vigo, A.; Reichelt, A.; Duncan, B.B.; Schmidt, M.I. Fasting plasma glucose to avoid a full OGTT in the diagnosis of gestational diabetes. Diabetes Res. Clin. Pract. 2016, 105, 322–326.
- Plows, J.F.; Stanley, J.L.; Baker, P.N.; Reynolds, C.M.; Vickers, M.H. The Pathophysiology of Gestational Diabetes Mellitus. Int. J. Mol. Sci. 2018, 19, 3342.
- Chen, P.; Wang, S.; Ji, J.; Ge, A.; Chen, C.; Zhu, Y.; Xie, N.; Wang, Y. Risk factors and management of gestational diabetes. Cell Biochem. Biophys. 2016, 71, 689–694.
- Zhang, C.; Rawal, S.; Chong, Y.S. Risk factors for gestational diabetes: Is prevention possible? Diabetologia 2016, 59, 1385–1390.
- Carolan, M. Maternal age ≥45 years and maternal and perinatal outcomes: A review of the evidence. Midwifery 2013, 29, 479–489.
- Lean, S.C.; Derricott, H.; Jones, R.L.; Heazell, A.E.P. Advanced maternal age and adverse pregnancy outcomes: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0186287.
- Cho, Y.M.; Kim, T.H.; Lim, S.; Choi, S.H.; Shin, H.D.; Lee, H.K.; Park, K.S.; Jang, H.C. Type 2 diabetes-associated genetic variants discovered in the recent genome-wide association studies are related to gestational diabetes mellitus in the Korean population. Diabetologia 2009, 52, 253–261.
- Zhou, Y.; Xie, N.; Zhang, L.; Chen, D. Impact of family history of diabetes on blood glucose, lipid levels and perinatal outcomes in pregnant women with gestational diabetes mellitus. J. Zhejiang Univ. 2021, 50, 329–334.
- Lauenborg, J.; Grarup, N.; Damm, P.; Borch-Johnsen, K.; Jørgensen, T.; Pedersen, O.; Hansen, T. Common type 2 diabetes risk gene variants associate with gestational diabetes. J. Clin. Endocrinol. Metab. 2009, 94, 145–150.
- Urbanová, J.; Brunerová, L.; Nunes, M.A.; Brož, J. MODY diabetes and screening of gestational diabetes. Ceska Gynekol. 2020, 85, 124–130.
- Pu, J.; Zhao, B.; Wang, E.J.; Nimbal, V.; Osmundson, S.; Kunz, L.; Popat, R.A.; Chung, S.; Palaniappan, L.P. Racial/Ethnic Differences in Gestational Diabetes Prevalence and Contribution of Common Risk Factors. Paediatr. Perinat. Epidemiol. 2015, 29, 436–443.
- Jaffe, A.; Giveon, S.; Rubin, C.; Novikov, I.; Ziv, A.; Kalter-Leibovici, O. Gestational diabetes risk in a multi-ethnic population. Acta Diabetol. 2020, 57, 263–269.
- Schwartz, N.; Nachum, Z.; Green, M.S. The prevalence of gestational diabetes mellitus recurrence—Effect of ethnicity and parity: A metaanalysis. Am. J. Obstet. Gynecol. 2015, 213, 310–317.
- Shang, M.; Dong, X.; Hou, L. Correlation of adipokines and markers of oxidative stress in women with gestational diabetes mellitus and their newborns. J. Obstet. Gynaecol. Res. 2018, 44, 637–646.
- LifeCycle Project-Maternal Obesity and Childhood Outcomes Study Group. Association of Gestational Weight Gain with Adverse Maternal and Infant Outcomes. JAMA 2019, 321, 1702–1715.
- Barakat, R.; Refoyo, I.; Coteron, J.; Franco, E. Exercise during pregnancy has a preventative effect on excessive maternal weight gain and gestational diabetes. A randomized controlled trial. Braz. J. Phys. Ther. 2019, 23, 148–155.
- Zhang, C.; Schulze, M.B.; Solomon, C.G.; Hu, F.B. A prospective study of dietary pattRNS, meat intake and the risk of gestational diabetes mellitus. Diabetologia 2006, 49, 2604–2613.
- Badon, S.E.; Wartko, P.D.; Qiu, C.; Sorensen, T.K.; Williams, M.A.; Enquobahrie, D.A. Leisure Time Physical Activity and Gestational Diabetes Mellitus in the Omega Study. Med. Sci. Sports Exerc. 2016, 48, 1044–1052.
- Mijatovic-Vukas, J.; Capling, L.; Cheng, S.; Stamatakis, E.; Louie, J.; Cheung, N.W.; Markovic, T.; Ross, G.; Senior, A.; Brand-Miller, J.C.; et al. Associations of Diet and Physical Activity with Risk for Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis. Nutrients 2018, 10, 698.
- Lau, C.; Thibodeaux, J.R.; Hanson, R.G.; Narotsky, M.G.; Rogers, J.M.; Lindstrom, A.B.; Strynar, M.J. Effects of perfluorooctanoic acid exposure during pregnancy in the mouse. Toxicol. Sci. 2006, 90, 510–518.
- Fenton, S.E.; Reiner, J.L.; Nakayama, S.F.; Delinsky, A.D.; Stanko, J.P.; Hines, E.P.; White, S.S.; Lindstrom, A.B.; Strynar, M.J.; Petropoulou, S.E. Analysis of PFOA in dosed CD-1 mice. Part 2. Disposition of PFOA in tissues and fluids from pregnant and lactating mice and their pups. Reprod. Toxicol. 2009, 27, 365–372.
- Steenland, K.; Tinker, S.; Shankar, A.; Ducatman, A. Association of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) with uric acid among adults with elevated community exposure to PFOA. Environ. Health Perspect. 2010, 118, 229–233.
- Zhang, C.; Sundaram, R.; Maisog, J.; Calafat, A.M.; Barr, D.B.; Buck Louis, G.M. A prospective study of prepregnancy serum concentrations of perfluorochemicals and the risk of gestational diabetes. Fertil. Steril. 2015, 103, 184–189.
- Eriksen, K.T.; Raaschou-Nielsen, O.; McLaughlin, J.K.; Lipworth, L.; Tjønneland, A.; Overvad, K.; Sørensen, M. Association between plasma PFOA and PFOS levels and total cholesterol in a middle-aged Danish population. PLoS ONE 2013, 8, e56969.
- Wang, H.; Yang, J.; Du, H.; Xu, L.; Liu, S.; Yi, J.; Qian, X.; Chen, Y.; Jiang, Q.; He, G. Perfluoroalkyl substances, glucose homeostasis, and gestational diabetes mellitus in Chinese pregnant women: A repeat measurement-based prospective study. Environ. Int. 2018, 114, 12–20.
- Kim, M.K.; Han, K.; You, S.Y.; Kwon, H.S.; Yoon, K.H.; Lee, S.H. Prepregnancy smoking and the risk of gestational diabetes requiring insulin therapy. Sci. Rep. 2020, 10, 13901.
- Bar-Zeev, Y.; Haile, Z.T.; Chertok, I.A. Association between Prenatal Smoking and Gestational Diabetes Mellitus. Obstet. Gynecol. 2020, 135, 91–99.
- Hinkle, S.N.; Bao, W.; Wu, J.; Sun, Y.; Ley, S.H.; Tobias, D.K.; Qian, F.; Rawal, S.; Zhu, Y.; Chavarro, J.E.; et al. Association of Habitual Alcohol Consumption with Long-term Risk of Type 2 Diabetes Among Women With a History of Gestational Diabetes. JAMA Netw. Open 2021, 4, e2124669.
- Elting, M.W.; Korsen, T.J.M.; Bezemer, P.D.; Schoemaker, J. Prevalence of diabetes mellitus, hypertension and cardiac complaints in a follow-up study of a Dutch PCOS population. Hum. Reprod. 2001, 16, 556–560.
- Rojas, J.; Chávez-Castillo, M.; Bermúdez, V. O papel da metformina nos distúrbios metabólicos durante a gravidez: Síndrome dos Ovários Policísticos e Diabetes Mellitus Gestacional. Int. J. Reprod. Med. 2014, 2014, 797681.
- Rizzo, G.; Garzon, S.; Fichera, M.; Panella, M.M.; Catena, U.; Schiattarella, A.; de Franciscis, P.; Vilos, G.; Tesarik, J.; Török, P.; et al. Vitamin D and Gestational Diabetes Mellitus: Is There a Link? Antioxidants 2019, 8, 511.
- Wang, L.; Zhang, C.; Song, Y.; Zhang, Z. Deficiência de vitamina D no soro e risco de diabetes mellitus gestacional: Uma meta-análise. Arch. Med. Sci. 2020, 16, 742–751.
- Barker, D.J.; Bull, A.R.; Osmond, C.; Simmonds, S.J. Fetal and placental size and risk of hypertension in adult life. BMJ 1990, 301, 259–262.
- Yan, J.; Yang, H. Gestational diabetes mellitus, programing and epigenetics. J. Matern. Fetal Neonatal Med. 2014, 27, 1266–1269.
- Franzago, M.; Fraticelli, F.; Stuppia, L.; Vitacolonna, E. Nutrigenetics, epigenetics and gestational diabetes: Consequences in mother and child. Epigenetics 2019, 14, 215–235.
- Xia, Q.; Cai, H.; Xiang, Y.B.; Zhou, P.; Li, H.; Yang, G.; Jiang, Y.; Shu, X.O.; Zheng, W.; Xu, W.H. Prospective cohort studies of birth weight and risk of obesity, diabetes, and hypertension in adulthood among the Chinese population. J. Diabetes 2019, 11, 55–64.
- Mendonça, E.L.S.S.; de Lima Macêna, M.; Bueno, N.B.; de Oliveira, A.C.M.; Mello, C.S. Premature birth, low birth weight, small for gestational age and chronic non-communicable diseases in adult life: A systematic review with meta-analysis. Early Hum. Dev. 2020, 149, 105154.
- Szmuilowicz, E.D.; Josefson, J.L.; Metzger, B.E. Gestational Diabetes Mellitus. Endocrinol. Metab. Clin. N. Am. 2019, 48, 479–493.
- Kamana, K.C.; Shakya, S.; Zhang, H. Gestational diabetes mellitus and macrosomia: A literature review. Ann. Nutr. Metab. 2015, 66 (Suppl. 2), 14–20.
- Chiefari, E.; Arcidiacono, B.; Foti, D.; Brunetti, A. Gestational diabetes mellitus: An updated overview. J. Endocrinol. Investig. 2017, 40, 899–909.
- Dirar, A.M.; Doupis, J. Gestational diabetes from A to Z. World J. Diabetes 2017, 8, 489–511.
- Gascho, C.L.; Leandro, D.M.; e Silva, T.R.; Silva, J.C. Predictors of cesarean delivery in pregnant women with gestational diabetes mellitus. Rev. Bras. Ginecol. Obstet. 2017, 39, 60–65.
- Pedersen, J. Weight and length at birth of infants of diabetic mothers. Acta Endocrinol. 1954, 16, 330–342.
- Freinkel, N. Banting Lecture 1980. Of pregnancy and progeny. Diabetes 1980, 29, 1023–1035.
- Moore, T.R. A comparison of amniotic fluid fetal pulmonary phospholipids in normal and diabetic pregnancy. Am. J. Obstet. Gynecol. 2002, 186, 641–650.
- McFarland, L.V.; Raskin, M.; Daling, J.R.; Benedetti, T.J. Erb/Duchenne’s palsy: A consequence of fetal macrosomia and method of delivery. Obstet. Gynecol. 1986, 68, 784–788.
- Vaquero, G.; Ramos, A.; Martinez, J.C.; Valero, P.; Nunez-Enamorado, N.; Simon-De Las Heras, R.; Camacho-Salas, A. Paralisis braquial obstetrica: Incidencia, seguimiento evolutivo y factores pronosticos . Rev. Neurol. 2017, 65, 19–25.
- Araújo Júnior, E.; Peixoto, A.B.; Zamarian, A.C.; Elito Júnior, J.; Tonni, G. Macrosomia. Best Pract. Res. Clin. Obstet. Gynaecol. 2017, 38, 83–96.
- Silva, L.; Plösch, T.; Toledo, F.; Faas, M.M.; Sobrevia, L. Adenosine kinase and cardiovascular fetal programming in gestational diabetes mellitus. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165397.
- Fonseca, D.L. Morbidity and mortality in Brazil. Cad. Saúde Colet. 2015, 23, 1.
- Lowe, W.L.; Scholtens, D.M.; Lowe, L.P.; Kuang, A.; Nodzenski, M.; Talbot, O.; Catalano, P.M.; Linder, B.; Brickman, W.J.; Clayton, P.; et al. Association of Gestational Diabetes with Maternal Disorders of Glucose Metabolism and Childhood Adiposity. JAMA 2018, 320, 1005–1016.
- Prates, T. Nutrição no Início da Vida, Epigenética e Prevenção das Doenças Crônicas não Transmissíveis: Uma Janela de Oportunidades para Pediatras/São Paulo: ILSI Brasil—International Life Sciences Institute do Brasil: São Paulo, Brasil, 2018. Available online: https://ilsibrasil.org/wp-content/uploads/sites/9/2019/02/Fasc%C3%ADculo-EPIGEN%C3%89TICA.pdf (accessed on 15 October 2021).
- Ponzo, V.; Fedele, D.; Goitre, I.; Leone, F.; Lezo, A.; Monzeglio, C.; Finocchiaro, C.; Ghigo, E.; Bo, S. Diet-Gut Microbiota Interactions and Gestational Diabetes Mellitus (GDM). Nutrients 2019, 11, 330.
- Canfora, E.E.; Jocken, J.W.; Blaak, E.E. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat. Rev. Endocrinol. 2015, 11, 577–591.
- Clarke, G.; Stilling, R.M.; Kennedy, P.J.; Stanton, C.; Cryan, J.F.; Dinan, T.G. Minireview: Gut microbiota: The neglected endocrine organ. Mol. Endocrinol. 2014, 28, 1221–1238.
- Meijnikman, A.S.; Gerdes, V.E.; Nieuwdorp, M.; Herrema, H. Evaluating Causality of Gut Microbiota in Obesity and Diabetes in Humans. Endocr. Rev. 2018, 39, 133–153.
- Sircana, A.; Framarin, L.; Leone, N.; Berrutti, M.; Castellino, F.; Parente, R.; De Michieli, F.; Paschetta, E.; Musso, G. Altered Gut Microbiota in Type 2 Diabetes: Just a Coincidence? Curr. Diab. Rep. 2018, 18, 98.
- Hu, C.; Wong, F.S.; Wen, L. Type 1 diabetes and gut microbiota: Friend or foe? Pharmacol. Res. 2015, 98, 9–15.
- Crusell, M.K.W.; Hansen, T.H.; Nielsen, T.; Allin, K.H.; Rühlemann, M.C.; Damm, P.; Vestergaard, H.; Rørbye, C.; Jørgensen, N.R.; Christiansen, O.B.; et al. Gestational diabetes is associated with change in the gut microbiota composition in third trimester of pregnancy and postpartum. Microbiome 2018, 6, 89.
- Serino, M.; Fernández-Real, J.M.; García-Fuentes, E.; Queipo-Ortuño, M.; Moreno-Navarrete, J.M.; Sánchez, A.; Burcelin, R.; Tinahones, F. The gut microbiota profile is associated with insulin action in humans. Acta Diabetol. 2013, 50, 753–761.
- Crommen, S.; Simon, M.C. Microbial Regulation of Glucose Metabolism and Insulin Resistance. Genes 2017, 9, 10.
- Bao, W.; Bowers, K.; Tobias, D.K.; Olsen, S.F.; Chavarro, J.; Vaag, A.; Kiely, M.; Zhang, C. Prepregnancy low-carbohydrate dietary pattern and risk of gestational diabetes mellitus: A prospective cohort study. Am. J. Clin. Nutr. 2014, 99, 1378–1384.
- Mokkala, K.; Tertti, K.; Rönnemaa, T.; Vahlberg, T.; Laitinen, K. Evaluation of serum zonulin for use as an early predictor for gestational diabetes. Nutr. Diabetes 2017, 7, e253.
- Jayashree, B.; Bibin, Y.S.; Prabhu, D.; Shanthirani, C.S.; Gokulakrishnan, K.; Lakshmi, B.S.; Mohan, V.; Balasubramanyam, M. Increased circulatory levels of lipopolysaccharide (LPS) and zonulin signify novel biomarkers of proinflammation in patients with type 2 diabetes. Mol. Cell. Biochem. 2014, 388, 203–210.
- Cortez, R.V.; Taddei, C.R.; Sparvoli, L.G.; Ângelo, A.G.S.; Padilha, M.; Mattar, R.; Daher, S. Microbiome and its relation to gestational diabetes. Endocrine 2019, 64, 254–264.
- Wang, J.; Zheng, J.; Shi, W.; Du, N.; Xu, X.; Zhang, Y.; Ji, P.; Zhang, F.; Jia, Z.; Wang, Y.; et al. Dysbiosis of maternal and neonatal microbiota associated with gestational diabetes mellitus. Gut 2018, 67, 1614–1625.
- Hasain, Z.; Mokhtar, N.M.; Kamaruddin, N.A.; Mohamed Ismail, N.A.; Razalli, N.H.; Gnanou, J.V.; Raja Ali, R.A. Gut Microbiota and Gestational Diabetes Mellitus: A Review of Host-Gut Microbiota Interactions and Their Therapeutic Potential. Front. Cell. Infect. Microbiol. 2020, 10, 188.
- Ferrocino, I.; Ponzo, V.; Gambino, R.; Zarovska, A.; Leone, F.; Monzeglio, C.; Goitre, I.; Rosato, R.; Romano, A.; Grassi, G.; et al. Changes in the gut microbiota composition during pregnancy in patients with gestational diabetes mellitus (GDM). Sci. Rep. 2018, 8, 12216.
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514.
- Gomes, A.C.; Bueno, A.A.; de Souza, R.G.; Mota, J.F. Gut microbiota, probiotics and diabetes. Nutr. J. 2014, 13, 60.
- Yan, F.; Cao, H.; Cover, T.L.; Whitehead, R.; Washington, M.K.; Polk, D.B. Soluble proteins produced by probiotic bacteria regulate intestinal epithelial cell survival and growth. Gastroenterology 2007, 132, 562–575.
- Banan, A.; Keshavarzian, A.; Zhang, L.; Shaikh, M.; Forsyth, C.B.; Tang, Y.; Fields, J.Z. NF-kappaB activation as a key mechanism in ethanol-induced disruption of the F-actin cytoskeleton and monolayer barrier integrity in intestinal epithelium. Alcohol 2007, 41, 447–460.
- Hajifaraji, M.; Jahanjou, F.; Abbasalizadeh, F.; Aghamohammadzadeh, N.; Abbasi, M.M.; Dolatkhah, N. Effect of probiotic supplements in women with gestational diabetes mellitus on inflammation and oxidative stress biomarkers: A randomized clinical trial. Asia Pac. J. Clin. Nutr. 2018, 27, 581–591.
- Kinalski, M.; Sledziewski, A.; Telejko, B.; Zarzycki, W.; Kinalska, I. Lipid peroxidation and scavenging enzyme activity in streptozotocin-induced diabetes. Acta Diabetol. 2000, 37, 179–183.
- Fugmann, M.; Breier, M.; Rottenkolber, M.; Banning, F.; Ferrari, U.; Sacco, V.; Grallert, H.; Parhofer, K.G.; Seissler, J.; Clavel, T.; et al. The stool microbiota of insulin resistant women with recent gestational diabetes, a high risk group for type 2 diabetes. Sci. Rep. 2015, 5, 13212.
- Karamali, M.; Dadkhah, F.; Sadrkhanlou, M.; Jamilian, M.; Ahmadi, S.; Tajabadi-Ebrahimi, M.; Jafari, P.; Asemi, Z. Effects of probiotic supplementation on glycaemic control and lipid profiles in gestational diabetes: A randomized, double-blind, placebo-controlled trial. Diabetes Metab. 2016, 42, 234–241.
- Kijmanawat, A.; Panburana, P.; Reutrakul, S.; Tangshewinsirikul, C. Effects of probiotic supplements on insulin resistance in gestational diabetes mellitus: A double-blind randomized controlled trial. J. Diabetes Investig. 2019, 10, 163–170.
- Babadi, M.; Khorshidi, A.; Aghadavood, E.; Samimi, M.; Kavossian, E.; Bahmani, F.; Mafi, A.; Shafabakhsh, R.; Satari, M.; Asemi, Z. The Effects of Probiotic Supplementation on Genetic and Metabolic Profiles in Patients with Gestational Diabetes Mellitus: A Randomized, Double-Blind, Placebo-Controlled Trial. Probiotics Antimicrob. Proteins 2019, 11, 1227–1235.
- Badehnoosh, B.; Karamali, M.; Zarrati, M.; Jamilian, M.; Bahmani, F.; Tajabadi-Ebrahimi, M.; Jafari, P.; Rahmani, E.; Asemi, Z. The effects of probiotic supplementation on biomarkers of inflammation, oxidative stress and pregnancy outcomes in gestational diabetes. J. Matern. Fetal Neonatal Med. 2018, 31, 1128–1136.
- Nabhani, Z.; Hezaveh, S.J.G.; Razmpoosh, E.; Asghari-Jafarabadi, M.; Gargari, B.P. The effects of symbiotic supplementation on insulin resistance/sensitivity, lipid profile and total antioxidant capacity in women with gestational diabetes mellitus: A randomized double blind placebo controlled clinical trial. Diabetes Res. Clin. Pract. 2018, 138, 149–157.
- Jamilian, M.; Amirani, E.; Asemi, Z. The effects of vitamin D and probiotic co-supplementation on glucose homeostasis, inflammation, oxidative stress and pregnancy outcomes in gestational diabetes: A randomized, double-blind, placebo-controlled trial. Clin. Nutr. 2019, 38, 2098–2105.
- Karamali, M.; Nasiri, N.; Taghavi Shavazi, N.; Jamilian, M.; Bahmani, F.; Tajabadi-Ebrahimi, M.; Asemi, Z. The Effects of Synbiotic Supplementation on Pregnancy Outcomes in Gestational Diabetes. Probiotics Antimicrob. Proteins 2018, 10, 496–503.
- Ahmadi, S.; Jamilian, M.; Tajabadi-Ebrahimi, M.; Jafari, P.; Asemi, Z. The effects of synbiotic supplementation on markers of insulin metabolism and lipid profiles in gestational diabetes: A randomised, double-blind, placebo-controlled trial. Br. J. Nutr. 2016, 116, 1394–1401.
- Jafarnejad, S.; Saremi, S.; Jafarnejad, F.; Arab, A. Effects of a Multispecies Probiotic Mixture on Glycemic Control and Inflammatory Status in Women with Gestational Diabetes: A Randomized Controlled Clinical Trial. J. Nutr. Metab. 2016, 2016, 5190846.
- Dolatkhah, N.; Hajifaraji, M.; Abbasalizadeh, F.; Aghamohammadzadeh, N.; Mehrabi, Y.; Abbasi, M.M. Is there a value for probiotic supplements in gestational diabetes mellitus? A randomized clinical trial. J. Health Popul. Nutr. 2015, 33, 25.
- Lindsay, K.L.; Brennan, L.; Kennelly, M.A.; Maguire, O.C.; Smith, T.; Curran, S.; Coffey, M.; Foley, M.E.; Hatunic, M.; Shanahan, F.; et al. Impact of probiotics in women with gestational diabetes mellitus on metabolic health: A randomized controlled trial. Am. J. Obstet. Gynecol. 2015, 212, e1–e11.
