Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Vivi Li and Version 1 by Mohammad Zaki Ahmad.
Breast cancer therapeutic intervention continues to be ambiguous owing to the lack of strategies for targeted transport and receptor-mediated uptake of drugs by cancer cells. In addition to this, sporadic tumor microenvironment, prominent restrictions with conventional chemotherapy, and multidrug-resistant mechanisms of breast cancer cells possess a big challenge to even otherwise optimal and efficacious breast cancer treatment strategies. Surface-modified nanomedicines can expedite the cellular uptake and delivery of drug-loaded nanoparticulate constructs through binding with specific receptors overexpressed aberrantly on the tumor cell.
- breast cancer
- multidrug resistance
- nanoparticle
- surface-modification
- receptor-mediated
- targeted delivery
1. Introduction
Breast cancer has an extensive history, dating back more than 3500 years to around 1500 B.C. when it was first mentioned by the ancient Egyptians [1]. Breast cancer remains the most commonly diagnosed cancer in women and the leading cause of cancer-related fatalities [2,3][2][3]. Breast cancer incidence has increased up to more than 30% in the previous 25 years, despite significant reductions in mortality [3,4][3][4]. According to WHO, in 2020, there were 2.3 million women diagnosed with breast cancer and 685,000 deaths globally [5]. Breast cancer had been diagnosed in 7.8 million women in the previous five years until the end of 2020, making it the most prevalent cancer worldwide [5]. Furthermore, as per WHO, breast cancer accounting for 12% of all new annual cancer cases globally and became the most prevalent form of cancer worldwide as of 2021 [6]. The primary risk factor for breast cancer includes gender, genetic factor, hormonal therapy, lifestyle and dietary habits, and growing age [3,6,7][3][6][7].
Breast cancer is a multifaceted disease characterized by a wide range of molecular profiles with characteristics of biological and clinical features [8]. Depending on the molecular characteristics, breast cancer is broadly classified into four types: (i) luminal A (ii) luminal B, (iii) human epidermal growth factor receptor 2 (HER2)-positive, (with overexpressed HER2 and negative estragon and progesterone receptors), and (iv) basal-like breast cancer (also known as hormone and HER2 negative or triple-negative breast cancer (TNBC)) [3,9][3][9]. Treatment options for breast cancer now include surgery (mastectomy and lumpectomy), radiation, chemotherapy, and hormone therapy. Chemotherapeutic options for breast cancer include tamoxifen (estrogen antagonist) for luminal A and luminal B, trastuzumab (antibodies) but chemotherapeutic options for TNBC are limited [3,7][3][7].
2. Nanotechnology-Mediated Drug Delivery in Breast Cancer
Most of the cancer chemotherapeutics are typically hydrophobic, nontargeted, and toxic in nature that can have serious adverse effects [36][10]. Over the last three decades, anthracyclines and taxanes have been two cornerstone chemotherapeutics utilized in advanced breast cancer chemotherapy [37][11]. However, cardiotoxicity has been linked to the anthracycline family, particularly doxorubicin formulations [38][12]. Similarly, taxanes have been reported to induce bone marrow depression, hypersensitivity reactions, and dose-dependent neurotoxicity [39,40,41][13][14][15]. In recent decades, advancement in the therapeutic approach of breast cancer has been significantly improved by the surface-modified nanomedicine. Nanotechnology provides a potential approaches alternative to conventional chemotherapeutics, conquering MDR by encapsulation or conjugating therapeutic moieties to nanometric carriers [10,11,12,13,16,18][16][17][18][19][20][21]. The emerging nanomedicine provides desirable drug delivery features, including solubilized lipophilic therapeutic agents, improved biocompatibility, diminished drug degradation in blood, reduced drug clearance, enhanced passive or active targeting, significantly decreased nonspecific cellular internalization, and negligible side effects [15,16,42,43][22][20][23][24]. Additionally, drug-loaded nanocarriers have recently proved improved treatment efficacy against MDR breast cancer and reduced detrimental side effects, when compared to traditional, nontargeted therapeutic drugs either single or in combination. Furthermore, surface-modified nanomedicine also has the potential to successfully target and remove breast cancer stem cells, which may play a significant role in breast cancer instigation, recurrence, and chemo/radiotherapy resistance [44][25]. Hence, rapidly evolving nanotechnology has the ability to address the intricate and multifaceted mechanisms of MDR, allowing nanomedicine to offer a novel and promising approach to combat and conquer chemoresistance against breast cancer. With the rapid growth of nanotechnology over the last 30 years, numerous nanomaterials have been developed, but only a few nanocarrier-based systems are being used in nanomedicine, and even fewer can fulfill the approval criteria of the US FDA [15][22]. The nanocarrier system can be broadly classified as lipid-based, polymeric, inorganic, and a hybrid of lipid and polymeric nanoparticles (Figure 31) [10,11,12,13,15,16,18][16][17][18][19][22][20][21].
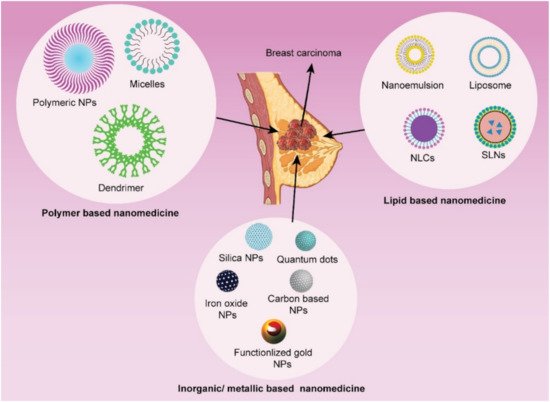

Figure 31. Different types of nanoparticulate systems utilized in receptor-mediated targeted drug delivery in breast cancer.
The lipid-based nanocarriers have been proved to be superior over polymeric and inorganic nanocarriers in terms of biocompatibility, safety, and biodegradability. Lipid nanocarrier drug delivery system can be sub-categorized into nanoemulsion, self-nano emulsifying drug delivery system (SNEDDS), liposomes, solid lipid nanoparticles (SLN), and nanostructured lipid carrier (NLC). In SNEDDS and nanoemulsion (oil-in-water), the modifying agent such as probes or imaging agents and lipophilic anticancer drugs are enclosed in oil droplets for specific tumor site delivery. In liposomes, the imaging/diagnostic agent can be enclosed with anticancer therapeutics either in an aqueous core or lipid bilayer according to their physicochemical characteristics (hydrophilicity/lipophilicity). Solid matrix lipid nanoparticles such as SLN (composed of solid lipid only) and NLC (composed of both solid and liquid lipid) entrapped the lipophilic therapeutics and imaging agents into the lipid phase of this delivery system. The various attributes of liposomes (like unique structure, and great flexibility to accommodate both hydrophilic and lipophilic therapeutic/imaging agents) make it promising therapeutic/imaging compared to other lipidic nanocarrier systems. However, optimizing lipid nanomedicines with desirable size distribution, shape, surface charge, and stability is quite challenging in itself. Recently, exploring cancer cell-specific drug delivery to improve the antitumor efficacy of lipid-based nanocarriers by surface functionalization with various ligands and peptides is contemplated in extensive research work. These lipid nanocarriers are now being designed to encapsulate or conjugate multifunctional components such as anticancer agents, targeting moieties, antibodies, etc. in one single formulation, therefore can be called complex nanoproducts [10,11,12,13,15,16,18][16][17][18][19][22][20][21]. Inorganic nanoparticles have also been widely investigated for the delivery of anticancer therapeutics and have demonstrated encouraging findings in pre-clinical studies that prompt for their translation clinical trials. Gold nanoparticles, quantum dots, superparamagnetic iron oxides are examples of inorganic nanoparticles. Whereas carbon nanotubes, polymeric micelles, dendrimers, nanoemulgel, mesoporous silica nanoparticles, metallic nanoparticles, and polymer-drug conjugated are the potential polymeric or organic nanocarriers. Surface modification of these lipid-based, organic and inorganic nanoparticles with targeting moieties, ligands, or other compounds is possible, providing these nanocarriers multifunctional properties [10,11,12,13,15,16,18,19,43,45][16][17][18][19][22][20][21][26][24][27].
Lipid-polymer hybrid is a promising new generation nanocarrier system and concept has originated from the fusion of liposomes and polymeric nanoparticles. It has polymeric core enclosed by a similar kind of lipid bilayer shell. Many researchers are exploring its potential as drug delivery nanocarrier system particularly for anticancer therapeutics in current scenario [44,45][25][27].
Among the plethora of potential nanocarriers, liposomes are the most widely explored nanocarrier and have also been established as an anticancer nanomedicine in the form of Doxil®. It has been used against Kaposi’s sarcoma, refractory breast and ovarian cancer with a circulating half-life of 74 h in breast cancer patients, compared to approximately 5 min t1/2 for free drug [46][28]. The prolonged circulation half-life of Doxil® is attributed to pegylation (coating of liposome surface with polyethylene glycol) which guards the liposomes against recognition by the mononuclear phagocyte system and offers a stabilization effect by mitigating adherence to cells, blood vessel walls, and other surfaces. Furthermore, it was also observed that the risk of cardiotoxicity associated with a peak concentration of drug was significantly reduced with Doxil® in breast cancer patients in comparison to cancer patients who developed cardiotoxicity when treated with free drug [47][29]. Liposomes, as nanocarriers, can be tailored the way they can offer passive or active targeting, and thereby overcome MDR and reduce side effects of drugs. The regulation of P-gp by liposomes is another key strategy for improving anticancer medication and therapeutic efficacy in MDR cancer cells [48][30]. Polymeric micelles represent another kind of potential nanocarriers system with promising delivery of chemotherapeutic agents to both nonresistant and MDR cancer cells [49][31]. A polymeric micelle is formed primarily when the hydrophobic section of a block copolymer is forced into the aqueous phase, while the hydrophilic portion faces outward to form a shell. Nanoemulsions are another kind of nanocarriers that are being explored as an anticancer drug delivery system in MDR [50][32]. Most of the nanoemulsions are oil in water types, in which oil droplets are dispersed in the continuous phase (water) at the size of about 10–100 nm. Because of their great solubilization capacity for lipophilic drugs in their oily phase, the oil-in-water type is the most widely used emulsion system. Among nanoparticulate systems, polymer nanoparticles, solid lipid nanoparticles, inorganic nanoparticles, polymeric conjugates, carbon nanotubes, and dendrimers have all been extensively investigated for their potential to overcome MDR in breast cancer.
3. Surface-Modified Multifunctional Nanomedicine for Breast Cancer
The current focus in pharmaceutical development and innovation is shifting towards the ‘smart drug’ paradigm, in which improved efficacy and minimal toxicity are of prime significance. This could be attained through delivering the cancer chemotherapeutics by targeting tumor-specific receptors. Identification of particular surface receptors in cancer cells that allows targeted delivery of cancer chemotherapeutics or other therapeutic agents like small interfering RNA (siRNA), can remarkably minimize the undesirable effects and upsurge the efficacy of cancer therapy [51,52][33][34]. Many breast cancer cell markers act as potential targets for the precise delivery of cancer chemotherapeutics which include overexpressed estrogen receptor (ER), progesterone receptor, HER2 receptor, folate receptor (FR), epidermal growth factor receptor (EGFR), transferrin receptor (TfR), integrin receptor, nucleolin receptor, CD44 receptor, etc. on the surface of breast cancer cells [53,54][35][36]. These receptors are highly overexpressed on the breast cancer cells as compared to normal cells and serve as a captivating way to deliver many potent anticancer agents loaded nanoparticulate system [55,56][37][38]. Various receptor-specific therapeutics utilized in breast cancer treatment are presented graphically in Figure 42. The major advantages of receptor-mediated active targeting in breast cancer are (i) it can prevent or at least substantially limit untoward effects of the anticancer agent on healthy tissues; (ii) it can enhance internalization loaded therapeutics by cancer cells; and (iii) it can overcome (at least in part) resistance mechanisms that are based on the active efflux of loaded therapeutics from cancer cells [57][39].
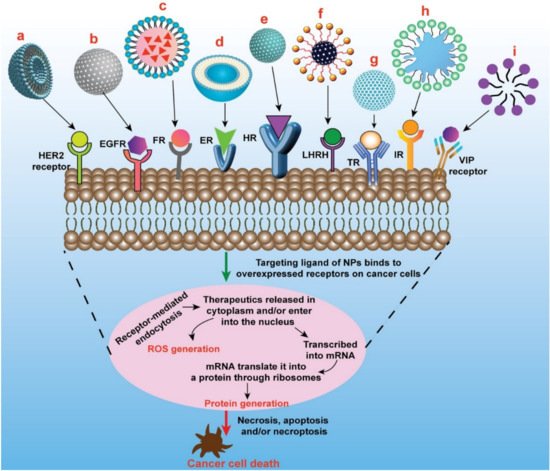

Figure 42. Schematic presentation of different types of targeted nanomedicine mediated through overexpressed receptors in breast cancer to induce cancer cell death/apoptosis. a. Oligoclonal antibody conjugated liposomes loaded with doxorubicin induce significant cell killing in HER2-overexpressed BT-474 breast cancer cells. b. GE11 peptides conjugated PEGylated PLGA nanoparticles loaded with curcumin induce significant cell killing in EGFR-overexpressed MCF-7 breast cancer cells c. Folate conjugated nanostructured lipid carriers loaded with curcumin induce significant inhibition of tumor growth compared to nontargeted nanomedicine in MCF-7 xenograft mice model. d. Doxorubicin-loaded liposomes surface-grafted with tamoxifen (ER antagonist) cause increased cellular/nuclear uptake of loaded therapeutics and induce more cell death compared to plain liposomes in ER overexpressed MCF-7 cells. e. PLGA/HA copolymers nanoparticles loaded with docetaxel causes increased cellular uptake and cytotoxicity in MDA-MB-231 breast cancer cells through CD44-mediated endocytosis. f. LHRH-conjugated PEGylated magnetite nanoparticles exhibited enhanced uptake in triple-negative breast cancer cells. g. Transferrin-capped mesoporous silica nanoparticles loaded with doxorubicin cause increased internalization in MCF-7 cells through transferrin-mediated endocytosis. h. Doxorubicin-loaded leukocyte mimicking nanoformulation (leukosomes) exhibited enhanced accumulation in tumor and significantly reduces the tumor volume. i. VIP functionalized phospholipid micelles loaded with pararubicin cause increased anticancer activity in MCF- 7 cells. HER2—Human Epidermal Growth Factor Receptor2; EGFR—Epidermal Growth Factor Receptor (HER1); FR—Folate Receptor; ER—Estrogen Receptor; HR—CD44/Hyaluronan Receptor; LHRH—Luteinizing Hormone-Releasing Hormone Receptor; IR—Integrin Receptor; VIP—Vasoactive Intestinal Peptide Receptor.
Although receptor-targeted nanomedicine is being explored for a long time, yet it is challenging to find new ideal ligands that could fulfill the pre-requisites of the targeted drug delivery. For receptor-targeted nanomedicine, the ligand should exhibit specificity as well as a high binding affinity towards its receptor. The density of ligands on the nanocarriers is important for optimal interaction with the receptors. If the density of the ligand is too high, the binding efficiency may reduce owing to steric hindrance. The ligand should have appropriate functional groups that can be modified chemically for conjugation onto the drug-loaded nanocarriers without affecting its receptor binding affinity. The receptor should facilitate the internalization of nanocarriers for intracellular delivery of active cytotoxic agents. These intricate requirements are the main challenges in taking receptor-targeted nanomedicine to the clinical stage [58,59][40][41].
3.1. Targeting of ErbB Receptor
Breast tumors express elevated levels of growth factors, and their receptors and breast cancer cells show either autocrine or paracrine-stimulated growth [60][42]. The ErbB tyrosine kinase receptors (type I tyrosine kinases receptors) are widely investigated growth factor receptor systems in breast cancer. This receptor system consists of four homolog receptors: ErbB1 (HER1/EGFR), ErbB2 (HER2/neu), ErbB3 (HER3), and ErbB4 (HER4) [61,62,63][43][44][45]. EGFR receptor is overexpressed in 15–20% of breast cancer cells and HER2 receptor is overexpressed in about 20–25% of breast cancer cells [64][46]. The overexpressed ErbB receptors show aggressive clinical behavior on breast carcinoma. Due to these reasons, treatments focusing on these receptors have the potential to be valuable anticancer therapies [65][47].
Farasat and coworkers developed oligoclonal antibody conjugated DOX-loaded liposomes and compared its efficiency with nonconjugated liposomes in targeting HER2-overexpressing and HER-2 negative breast cancer cell lines. In the results, scientists observed that oligoclonal antibody conjugated DOX-loaded liposomes showed an increased binding efficiency to HER2 overexpressing BT-474 and SK-BR-3 breast tumor cells and HER2-negative human breast epithelial MCF-10A cell lines. The results showed receptor-specific binding of targeted liposomes to SK-BR-3 and BT474 cells. This could be due to liposomes conjugated with oligoclonal antibodies compared to monoclonal antibodies-conjugated liposomes. However, oligoclonal antibody conjugated liposomes exhibited higher cytotoxicity in HER2-positive tumor cells compared to the nontargeted liposomes [66][48]. In another study, Duan et al. developed Fab’ (antigen-binding fragments cut from trastuzumab) and trastuzumab modified curcumin-loaded PEG -PLGA NPs (Fab′-Cur-NPs and TMAB-Cur-NPs). The in vitro results showed a prominent ability to kill HER2-overexpressing BT-474 breast cancer cells and significant accumulation compared to the untargeted NPs. However, there was no significant difference in the accumulation in HER2 negative MDA-MB-231 cells. A pharmacokinetic study in Sprague-Dawley rats showed an enhancement in the half-life (t1/2) and absolute bioavailability of Fab′-Cur-NPs by 5.30 and 1.76 folds respectively as compared to that of TMAB-Cur-NPs. Moreover, the biodistribution study showed that Fab′-Cur-NPs demonstrated significantly higher tumor accumulation compared to TMAB-Cur-NPs [67][49].
In a research investigation, EGFR-targeting GE11 peptides conjugated curcumin-loaded PLGA-PEG NPs demonstrated enhanced delivery of curcumin to EGFR-expressing MCF-7 cells compared to free curcumin and non-EGFR targeted NPs. Moreover, treatment of breast cancer cells and tumor-bearing mice with these EGFR targeted curcumin NPs led to decreased phosphoinositide 3-kinase signaling, a significant reduction in cancer cell viability, low drug clearance from the circulation, and significant suppression in tumor burden as compared with free curcumin and nontargeted NPs [68][50]. Herceptin-conjugated PLA-D-α-tocopheryl polyethylene glycol 1000 succinate (TPGS) blend NPs showed significantly higher cellular uptake in SK-BR-3 cells. In vitro cell line investigations demonstrated that targeted NPs had a 9.6-fold lower IC50 value in comparison with untargeted NPs [69][51]. Similarly, Kutty and Feng, developed cetuximab-conjugated TPGS micelles for targeted delivery of docetaxel to TNBC cells. In vitro investigations revealed cetuximab-conjugated TPGS micelles exhibited remarkably higher cellular uptake as compared to unconjugated NPs on MDA-MB-468 and MDA-MB-231 breast cancer cells which overexpressed EGFR on the cell surface. In vitro cytotoxicity study showed that cetuximab-conjugated TPGS micelles exhibited 205.6 and 223.8-folds higher efficiency compared to free drug solution against the MDA-MB-468 and MDA-MB-231 cell lines respectively [70][52].
Milane et al. synthesized an EGFR-targeted paclitaxel/lonidamine co-loaded PLGA/PEG/EGFR targeting peptide combined poly(ε-caprolactone) NPs for the treatment of MDR breast tumor cells. The peptide delivery system actively targeted MDR cells by exploiting the EGFR expression. The targeting system inhibited the Warburg effect and promoted mitochondrial binding of pro-apoptotic Bcl-2 proteins (by lonidamine), while hyper stabilizing the microtubules (by paclitaxel). This strategy enhanced the therapeutic index of both drugs and showed the potentiating effect of combination therapy in the treatment of MDR breast cancer [71][53]. In another study, Dilnawaz et al. developed HER2 antibody conjugated paclitaxel and/or rapamycin-loaded glycerol monooleate coated magnetic NPs which exhibited an enhanced uptake in the MCF-7 human breast cancer cell line. In vitro cytotoxicity in the same cell line showed that the targeted paclitaxel-loaded magnetic NPs exhibited ~24-fold reduction in IC50 value than native paclitaxel and ~3 folds lower IC50 value than untargeted paclitaxel-loaded magnetic NPs. In the case of rapamycin, the targeted magnetic NPs exhibited ~71-fold reduction in IC50 value than native drug and ~10 folds lower IC50 value than untargeted magnetic NPs. In the case of combined drug formulation, the targeted NPs exhibited ~55-fold reduction in IC50 value than native drugs and ~7-folds lower IC50 value than untargeted NPs. Therefore, antibody-doped NPs could be used as a promising drug carrier for the delivery of active therapeutic agents in targeted breast cancer therapy [72][54].
Acharya et al. developed EGFR antibody conjugated rapamycin-loaded polymeric PLGA NPs which showed that EGFR conjugated immunonanoparticles exhibited nearly 13-folds higher cellular uptake and antiproliferative activity than unconjugated NPs in MCF-7 cell line [73][55]. In another study, Sun et al. demonstrated that coumarin 6-loaded PLGA/montmorillonite-trastuzumab NPs exhibited significantly higher cellular uptake efficiency than the nontargeted NPs. The outcome of in vitro cytotoxicity investigation on SK-BR-3 cells further proved the targeting effects of the antibody conjugation. The therapeutic effect of the HER2 decorated NPs was 12.74-fold and 13.11-fold greater than that of the untargeted NPs and higher than taxols in terms of IC50 value, respectively [74][56].
These studies conclude that nanotechnology-based targeting of antineoplastic agents against ErbB receptors can be further explored to enhance their therapeutic efficacy against various breast cancers.
3.2. Targeting of Folate Receptor
Folic acid (vitamin B9) is water-soluble, low-molecular-weight (441.4 g/mol) compound that is essential in normal mitotic cell division and growth. Folic acid is very significant in infancy and pregnancy; however, it could also participate in nourishing some of the cancers [75][57]. Folic acid (FA) or folate has a very high affinity towards FR and FRs (especially the α-isoform) are expressed in a relatively higher percentage in breast cancer cells therefore these receptors can be successfully implicated in the fabrication of FR-mediated targeted drug delivery systems [76,77][58][59]. Folate conjugation has been largely exploited for receptor-targeted therapy of breast cancer [78][60]. FRs are cell surface glycosyl phosphatidylinositol anchored proteins that not only bind FA but also 5-methyltetrahydrofolate [79,80][61][62]. The FRs present on the cell surface binds the folate ligand and then the complexes are internalized into the cell through receptor-mediated endocytosis. Once internalized, these complexes release folate intracellularly in the endosomes, and the discharged FRs recycle back to the cell surface [81,82][63][64].
Folate-conjugated amphiphilic cyclodextrin paclitaxel NPs developed by scientists showed significantly higher cellular uptake and cytotoxicity in 4T1 breast cancer cells compared to untargeted NPs. Paclitaxel-loaded folate decorated NPs significantly reduced tumor burden and survival time compared to untargeted NPs [83][65]. Thapa et al. developed FA-decorated cisplatin, and docetaxel co-loaded liquid crystalline NPs, which exhibited significantly higher cellular uptake by FR-overexpressing MDA-MB-231 cells to a greater extent than FR-negative A549 cells in comparison to nontargeted NPs, attributed to folate receptor-mediated endocytosis of the targeted NPs. The increased expression of various apoptotic markers including Bax, p21, and cleaved caspase-3 along with improved antimigration effects in MDA-MB-231 breast cancer cells following treatment demonstrated that the folate decorated NPs can be used for successful treatment of metastatic breast cancer. Moreover, folate decorated NPs exhibited significantly higher anticancer efficacy both in vitro as well as in the MDA-MB-231 tumor xenograft model compared to nontargeted NPs [84][66].
Folate conjugated curcumin embedded nanostructured lipid carriers (FA-Cur-NLCs) showed significantly higher inhibition of tumor growth compared to curcumin NLCs and free curcumin solution in preclinical studies with MCF-7 tumor-bearing tumor xenografted Balb/c-nude mice model [85][67]. In another study, DOX-loaded FA-conjugated pluronic micelles significantly raised cellular uptake in MCF-7/MDR cells than untargeted micelles. It was also found that FA conjugated micelles showed ~3.3 and 8 folds higher reduction in tumor volume in 3 weeks in xenograft of MDR tumor-bearing Balb/c mice compared to untargeted micelles and free DOX solution, respectively (Figure 53) [86][68]. Gunduz et al. fabricated FA conjugated idarubicin-loaded magnetic NPs, which showed 90 folds higher uptake in MCF-7 cell lines compared to free idarubicin solution [87][69].
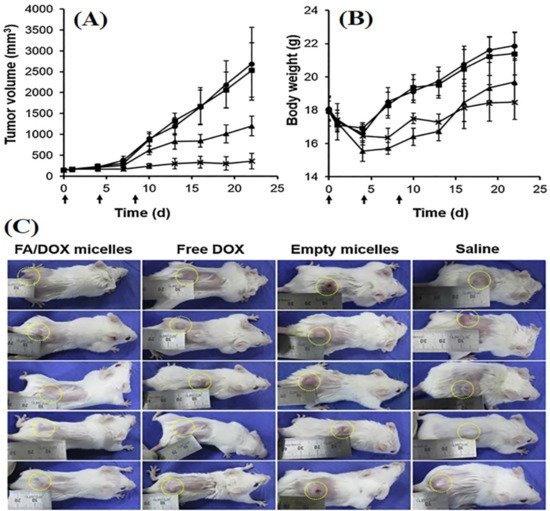

Figure 53. Image showing the tumor growth inhibition and impact on bodyweight of female Balb/c mice with MDR breast tumor (n = 5 ± SE) upon various treatment. Tumor-bearing mice were intravenously treated with normal saline (●), Pluronic (■), pure DOX (▲), and FA/DOX micelles (Χ) (dose of DOX = 4 mg/kg) at days 0, 4, and 8 (indicated by arrows). It indicated changes in (A) tumor volume; (B) body weight and (C) image showing MDR breast tumor-bearing Balb/c mice. The photographs of all the treated tumor-bearing mice were taken after 22 days of treatment. The tumors are indicated with yellow dotted circles. Reproduced with permission of Nguyen et al., Int. J. Pharm, published by Elsevier, 2015 [86][68].
Biodegradable FA conjugated deoxycholic acid-O-carboxymethylated chitosan NPs developed by Wang and coworkers showed significantly higher cellular uptake than untargeted NPs in MCF-7 breast cancer cells. It was also found that FA decorated NPs showed a significantly higher pro-apoptotic effect in MCF-7 cells due to overexpression of FRs on the surface of MCF-7 cells, which considerably increased the uptake of NPs through FR-mediated endocytosis [88][70]. Vincristine sulfate-loaded PLGA–PEG NPs decorated with FA designed and characterized by Chen et al. exhibited significantly higher cellular uptake than NPs without the FA decoration. PLGA–PEG–FA NPs exhibited 1.52- and 3.91-folds higher cytotoxicity on MCF-7 cells than that of NPs without folate and free vincristine sulfate, respectively [89][71].
FA conjugated nanocarriers are one of the most explored nanomedicines, investigated for delivery of the therapeutic agent, imaging agent as well as theranostic agents for treatment and diagnosis of cancer. The above studies demonstrated that folate-targeted nanomedicine could provide a promising strategy for the effective and precise treatment of breast cancer.
3.3. Targeting of Estrogen Receptor
Estrogen receptors (ER) belong to the nuclear hormone receptor superfamily, which is a class of transcription factors regulated by small ligands. These receptors are differentially overexpressed to 60–80% in breast cancer cells [90][72]. Estrogens are internalized into the cell after binding to ER receptor, therefore estrogens can be explored as a ligand for targeting tumor cells overexpressing ER receptors [91,92][73][74].
Estrone-targeted PEGylated paclitaxel and epirubicin co-loaded liposomal NPs showed a significantly higher cellular uptake, accumulation, and prolonged circulation time in both in vitro and in vivo investigations. Moreover, it was noticed that a significant suppression in the tumor growth was observed in animal model with no significant toxicity (Figure 64) [93][75].
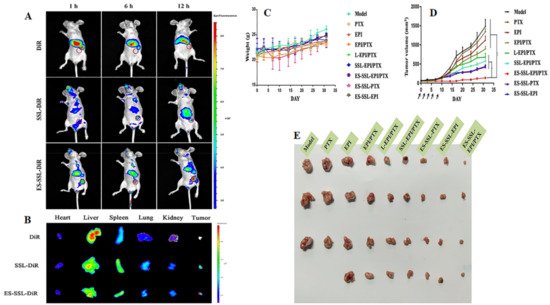

Figure 64. The photomicrograph showing the imaging of MCF-7 tumor-bearing Balb/c nude mice after intravenous administration of free DiR and DiR-labeled liposomes. (A) The in vivo imaging of Balb/c nude mice at predetermined time points; (B) The ex vivo imaging of different organs of Balb/c mice that were dissected just after 12 h of intravenous administration. The antitumor effect of ES-SSL-EPI/PTX in MCF-7 tumor-bearing Balb/c nude mice (n = 4). (C) Impact on body weights upon treatment; (D) Tumor volume after intravenous administration of free drugs and liposomal formulations; (E) The photomicrographs of MCF-7 tumors after intravenous administration of free drugs and liposomal formulations. ‘*’, ‘**’ represents the comparison with other treated groups, p < 0.05 and p < 0.01 respectively. Reproduced with permission of Tang et al., Int. J. Pharm, published by Elsevier, 2019 [93][75].
In another research, tamoxifen, an ER antagonist, surface-grafted liposomes loaded with DOX showed enhanced cellular and nuclear uptake of DOX in comparison to DOX-loaded liposomes and free DOX solution. In vitro investigations demonstrated that tamoxifen and DOX co-loaded liposomes were more cytotoxic to ER overexpressing MCF-7 cells as compared to DOX liposomes, DOX solution, and tamoxifen-DOX solution. Tamoxifen-DOX liposomes exhibited remarkably increased inhibition of tumor growth in animal model compared to DOX solution and DOX liposomes [94][76].
Estrone decorated DOX-loaded stealth liposomes exhibited significant cytotoxicity, accumulation, and antitumor efficacy compared to untargeted formulations in ER-positive MCF-7 tumor-bearing female Balb/c-nude mice [95][77]. In a previous study, tamoxifen-loaded poly (ethylene glycol)-thiol gold nanoparticle exhibited significantly higher cellular uptake and cytotoxicity in ERα(+) human squamous oral cancer HSC-3 and MCF-7 breast cancer cells compared to untargeted NPs [96][78]. Similarly, estrone decorated liposomes showed higher accumulation in the ER expressed breast tissue compared with the plain drug and conventional liposomes [91][73].
These studies demonstrated that estrone-targeted nanomedicine may advance the treatment of various estrogen receptor overexpressing breast cancers by utilizing receptor-mediated targeting of anticancer agents.
3.4. Targeting of CD44 Receptor/Hyaluronan Receptor
The glycosaminoglycan hyaluronan is a naturally occurring component of the extracellular matrix. Hyaluronic acid (HA) plays a crucial role in cell proliferation, migration, and invasion, and as these processes are involved in inflammation and cancer progression, therefore HA is significant in cancer pathophysiology. The HA receptor CD44 is found sparsely on the surface of epithelial, hematopoietic, and neuronal cells, and is abundantly overexpressed in various cancer cells [97,98,99][79][80][81]. CD44 regulates lymphocyte adhesion to endothelial cells during lymphocyte migration [100][82], a process that is comparable to solid tumor metastasis [101][83]. It is also involved in the regulation of the proliferation of cancer cells [102][84]. Recently, the cancer stem cell theory has proposed that CD44 can be employed as a marker of breast cancer stem cells [103,104][85][86]. The relationship between tumor cells and HA receptors indicates that it may be possible to exploit HA for active targeting to tumor cells bearing this receptor [105,106][87][88].
A novel redox-responsive and HA-functionalized chitosan lipoic acid NPs (HA-CSLA-NPs) loaded with cytoplasmic 17α-methyltestosterone showed significantly higher cellular internalization through CD44 receptors with rapid drug release, and enhanced cytotoxicity against CD44 overexpressing BT-20 breast cancer cell line as opposed to CD44 negative MCF-7 cell line [107][89]. Previously, Cerqueira and coworkers developed HA-modified PLGA NPs, which showed significantly enhanced cellular uptake and cytotoxicity against MDA-MB-231 breast cancer cells compared to non-HA-modified NPs. [108][90]. Liu et al. designed HA-decorated NLCs for co-delivery of baicalein and DOX for breast cancer therapy. In vitro cytotoxicity assay against MCF-7/ADR cells exhibited 2.25 and 12-fold reduction of IC50 value with HA decorated NLCs in comparison to baicalein and DOX loaded NLCs, and baicalein and DOX solution, respectively. In vivo antitumor efficacy study using Kunming mice bearing MCF-7/ADR breast cancer cells xenograft showed higher inhibition in tumor growth with targeted NLCs as compared to untargeted baicalein and DOX loaded NLCs, baicalein NLCs, DOX NLCs, and baicalein and free DOX solution [109][91].
Zhong et al. developed HA L-lysine methyl ester-lipoic acid (HA Lys-LA) NPs. These HA Lys-LA NPs exhibited 20-folds higher tumor uptake of DOX compared to free DOX. DOX-loaded crosslinked HA-Lys-LA NPs possessed significantly higher targetability and excellent antitumor activity towards CD44 positive MCF-7/ADR cells. Moreover, the developed NPs exhibited significantly greater survival of mice throughout the experimental period of 44 days and showed lesser side effects compared to untargeted NPs and free drugs [110][92]. In another study, Zhao et al. developed HA matrix NPs with intrinsic-CD44-tropism loaded with rapamycin which showed 3.2-fold drug uptake in CD-44 positive MD-MB-468 breast cancer cells compared to the free rapamycin. The study outcomes in vivo pharmacokinetics showed that HA decorated NPs exhibited a 2.96-fold increase in area under the curve (AUC) than that of the free drug, and the concomitant total body clearance was 8.82-fold slower than free drug [111][93].
Docetaxel-loaded self-assembled NPs of PLGA/HA block copolymers exhibited significantly higher uptake, enhanced cytotoxicity in MDA-MB-231 cells by CD44-mediated endocytosis compared to untargeted NPs and free drug. In vivo studies with Balb/c nude mice showed that HA decorated NPs exhibited significantly improved cancer targeting and anticancer activity compared to untargeted NPs and free drug [112][94]. Yang et al. developed HA oligosaccharide lipid NPs loaded with paclitaxel, which exhibited significantly enhanced antitumor response and activity of chemotherapies both in vitro and in vivo compared to nontargeted NPs [113][95].
These studies suggest that HA could be employed as a promising targeting ligand to target various nanomaterials carrying the anticancer payload to the CD44 receptor for the treatment of breast cancer.
3.5. Targeting of LHRH Receptor
Luteinizing hormone-releasing hormone (LHRH) is a hormonal decapeptide produced by the hypothalamus. It is also known as Gonadotropin-releasing hormone (GnRH). It plays an important role in the regulation of the pituitary-gonadal axis and reproduction. LHRH receptors are overexpressed in 80% of endometrial and ovarian, 86% of prostate, 50% of breast, and 80% of renal cancers [114][96]. These LHRH receptors are not expressed significantly in normal organs, therefore LHRH can act as a targeting ligand to improve the cellular uptake of anticancer drugs to LHRH receptor-positive cancerous cells like breast cancer cells and reduce the peripheral side effects [115][97].
Recently scientists conjugated LHRH with anticancer drugs, prodigiosin or paclitaxel, separately and evaluated these LHRH drug conjugates for treatment and specific targeting in MDA-MB-231 TNBC cells and tumor-bearing female nude mice. The designed conjugates showed promising efficiency to target TNBC cells specifically with the ability to cause significant regression in tumor growth without any significant toxicity [116][98]. Previously, scientists investigated the cellular uptake efficiency of LHRH-conjugated PEG-coated magnetite NPs (LMNPs) and PEG-coated magnetite NPs (MNPs) in normal breast cells and TNBC cells, and normal breast cells. In the result of this study, scientists observed enhanced uptake for LMNPs into TNBC cells attributed to the presence of LHRH on the surface. The entry of nanoparticle into breast cancer cells are also explored using a combination of thermodynamics and kinetics models [117][99]. The concepts of thermodynamics and kinetics models explain the interaction between a ligand decorated nanoparticulate system and receptors expressed over the cellular membrane. During the receptor-mediated endocytosis of the nanoparticulate system, cellular receptors bind to the ligands present on the nanoparticulate system to decrease the free energy of the system. It is proved in the current investigation that receptor-mediated endocytosis involves an interplay between thermodynamics and kinetics.
Varshosaz et al. developed magnetic LHRH chitosan bioconjugated NPs which demonstrated significantly enhanced cellular uptake and a 2-fold reduction in IC50 value against LHRH overexpressing MCF-7 cells compared to nontargeted NPs [118][100]. In another study, Li et al. developed cisplatin-loaded LHRH-modified dextran NPs which exhibited significantly higher uptake of cisplatin and cytotoxicity with a significant reduction in nephrotoxicity of cisplatin compared to nontargeted NPs. LHRH modified NPs exhibited a higher antitumor effect with low systemic toxicity compared to untargeted NPs in antitumor efficacy study in Balb/c mice bearing 4T1 tumors [119][101]. Furthermore, Taheri et al. developed LHRH conjugated methotrexate-human serum albumin NPs which exhibited about 7-fold higher antitumor efficacy compared to untargeted NPs. Additionally, LHRH NPs exhibited 216.66% improvement in the life span of mice [120][102].
The studies discussed above supports further exploration of LHRH conjugated nanomedicine for treatment and diagnosis of breast cancer including its most complicated version, TNBC. Thus, LHRH can be used as a potential targeting ligand for the targeted treatment of LHRH overexpressed breast cancers.
References
- Ebeid, N.I. Egyptian Medicine in the Days of the Pharaohs; General Egyptian Book Organization: Cairo, Egypt, 1999.
- Cardoso, D.; Coelho, A.; Fernandes, L.; Matos, L.V.; Serrano, I.; Miranda, H.; Martins, A. Sweet’s Syndrome Induced by Aromatase Inhibitor in the Treatment of Early Breast Cancer. Eur. J. Case Rep. Intern. Med. 2020.
- Rizwanullah, M.; Perwez, A.; Mir, S.R.; Rizvi, M.M.; Amin, S. Exemestane encapsulated polymer-lipid hybrid nanoparticles for improved efficacy against breast cancer: Optimization, in vitro characterization and cell culture studies. Nanotechnology 2021, 32, 415101.
- Sánchez-Jiménez, F.; Pérez, A.P.; de la Cruz-Merino, L.; Sánchez-Margalet, V. Obesity and Breast Cancer: Role of Leptin. Front. Oncol. 2019, 9, 596.
- WHO. Breast Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/breast-cancer (accessed on 17 September 2021).
- U.S. Breast Cancer Statistics|Breastcancer.org. Available online: https://www.breastcancer.org/symptoms/understand_bc/statistics (accessed on 17 September 2021).
- García-Aranda, M.; Redondo, M. Immunotherapy: A Challenge of Breast Cancer Treatment. Cancers 2019, 11, 1822.
- Perou, C.M.; Sørlie, T.; Eisen, M.B.; Van De Rijn, M.; Jeffrey, S.S.; Rees, C.A.; Pollack, J.R.; Ross, D.T.; Johnsen, H.; Akslen, L.A.; et al. Molecular portraits of human breast tumours. Nature 2000, 406, 747–752.
- Fragomeni, S.M.; Sciallis, A.; Jeruss, J.S. Molecular Subtypes and Local-Regional Control of Breast Cancer. Surg. Oncol. Clin. N. Am. 2018, 27, 95–120.
- Shapiro, C.L.; Recht, A. Side Effects of Adjuvant Treatment of Breast Cancer. N. Engl. J. Med. 2001, 344, 1997–2008.
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG); Peto, R.; Davies, C.; Godwin, J.; Gray, R.; Pan, H.C.; Clarke, M.; Cutter, D.; Darby, S.; McGale, P.; et al. Comparisons between different polychemotherapy regimens for early breast cancer: Meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet 2012, 379, 432–444.
- Smith, L.A.; Cornelius, V.R.; Plummer, C.J.; Levitt, G.; Verrill, M.; Canney, P.; Jones, A. Cardiotoxicity of anthracycline agents for the treatment of cancer: Systematic review and meta-analysis of randomised controlled trials. BMC Cancer 2010, 10, 337.
- Nurgalieva, Z.; Liu, C.-C.; Du, X.L. Chemotherapy use and risk of bone marrow suppression in a large population-based cohort of older women with breast and ovarian cancer. Med Oncol. 2010, 28, 716–725.
- Lee, C.; Gianos, M.; Klaustermeyer, W.B. Diagnosis and management of hypersensitivity reactions related to common cancer chemotherapy agents. Ann. Allergy Asthma Immunol. 2009, 102, 179–187.
- Hagiwara, H.; Sunada, Y. Mechanism of taxane neurotoxicity. Breast Cancer 2004, 11, 82–85.
- Ahmad, M.Z.; Akhter, S.; Jain, G.K.; Rahman, M.; Pathan, S.A.; Ahmad, F.J.; Khar, R.K. Metallic nanoparticles: Technology overview & drug delivery applications in oncology. Expert Opin. Drug Deliv. 2010, 7, 927–942.
- Akhter, S.; Ahmad, M.Z.; Singh, A.; Ahmad, I.; Rahman, M.; Anwar, M.; Jain, G.K.; Ahmad, F.; Khar, R.K. Cancer Targeted Metallic Nanoparticle: Targeting Overview, Recent Advancement and Toxicity Concern. Curr. Pharm. Des. 2011, 17, 1834–1850.
- Ahmad, M.Z.; Akhter, S.; Rahman, Z.; Akhter, S.; Anwar, M.; Mallik, N.; Ahmad, F. Nanometric gold in cancer nanotechnology: Current status and future prospect. J. Pharm. Pharmacol. 2012, 65, 634–651.
- Akhter, S.; Ahmad, M.Z.; Ahmad, F.; Storm, G.; Kok, R.J. Gold nanoparticles in theranostic oncology: Current state-of-the-art. Expert Opin. Drug Deliv. 2012, 9, 1225–1243.
- Akhter, M.H.; Rizwanullah, M.; Ahmad, J.; Ahsan, M.J.; Mujtaba, M.A.; Amin, S. Nanocarriers in advanced drug targeting: Setting novel paradigm in cancer therapeutics. Artif. Cells. Nanomed. Biotechnol. 2018, 46, 873–884.
- Ahmad, M.Z.; Ahmad, J.; Alasmary, M.Y.; Akhter, H.; Abdel-Wahab, B.A.; Warsi, M.H.; Haque, A. Progress in nanomedicine-based drug delivery in designing of chitosan nanoparticles for cancer therapy. Int. J. Polym. Mater. 2021, 1–22.
- Tang, X.; Loc, W.S.; Dong, C.; Matters, G.L.; Butler, P.J.; Kester, M.; Meyers, C.; Jiang, Y.; Adair, J.H. The use of nanoparticulates to treat breast cancer. Nanomedicine 2017, 12, 2367–2388.
- Yang, X.; Yi, C.; Luo, N.; Gong, C. Nanomedicine to Overcome Cancer Multidrug Resistance. Curr. Drug Metab. 2014, 15, 632–649.
- Rizwanullah, M.; Amin, S.; Mir, S.R.; Fakhri, K.U.; Rizvi, M.M. Phytochemical based nanomedicines against cancer: Current status and future prospects. J. Drug Target. 2018, 26, 731–752.
- Haider, N.; Fatima, S.; Taha, M.; Rizwanullah, M.; Firdous, J.; Ahmad, R.; Mazhar, F.; Khan, M.A. Nanomedicines in diagnosis and treatment of cancer: An update. Curr. Pharm. Des. 2020, 26, 1216–1231.
- Ahmad, J.; Akhter, S.; Khan, M.A.; Wahajuddin, M.; Greig, N.H.; Kamal, M.A.; Midoux, P.; Pichon, C. Engineered Nanoparticles Against MDR in Cancer: The State of the Art and its Prospective. Curr. Pharm. Des. 2016, 22, 4360–4373.
- Rizwanullah, M.; Alam, M.; Mir, S.R.; Rizvi, M.; Amin, S. Polymer-lipid hybrid nanoparticles: A next-generation nanocarrier for targeted treatment of solid tumors. Curr. Pharm. Des. 2020, 26, 1206–1215.
- O’Brien, M.E.R.; Wigler, N.; Inbar, M.; Rosso, R.; Grischke, E.; Santoro, A.; Catane, R.; Kieback, D.G.; Tomczak, P.; Ackland, S.P.; et al. Reduced cardiotoxicity and comparable efficacy in a phase IIItrial of pegylated liposomal doxorubicin HCl(CAELYX™/Doxil®) versus conventional doxorubicin forfirst-line treatment of metastatic breast cancer. Ann. Oncol. 2004, 15, 440–449.
- Coukos, G.; de Vries, J.; Hawkins, R.E. ESMO Symposium on Immuno-Oncology 2015 Officers and Organisation. Ann. Oncol. 2015, 26.
- Wang, X.; Wang, Q.; Liu, Z.; Zheng, X. Preparation, pharmacokinetics and tumour-suppressive activity of berberine liposomes. J. Pharm. Pharmacol. 2017, 69, 625–632.
- Gregoriou, Y.; Gregoriou, G.; Yilmaz, V.; Kapnisis, K.; Prokopi, M.; Anayiotos, A.; Strati, K.; Dietis, N.; Constantinou, A.I.; Andreou, C. Resveratrol loaded polymeric micelles for theranostic targeting of breast cancer cells. Nanotheranostics 2021, 5, 113–124.
- Abedinpour, N.; Ghanbariasad, A.; Taghinezhad, A.; Osanloo, M. Preparation of Nanoemulsions of Mentha piperita Essential Oil and Investigation of Their Cytotoxic Effect on Human Breast Cancer Lines. BioNanoScience 2021, 11, 428–436.
- Mohanty, C.; Das, M.; Kanwar, J.R.; Sahoo, S.K. Receptor Mediated Tumor Targeting: An Emerging Approach for Cancer Therapy. Curr. Drug Deliv. 2011, 8, 45–58.
- Large, D.E.; Soucy, J.; Hebert, J.; Auguste, D.T. Advances in Receptor-Mediated, Tumor-Targeted Drug Delivery. Adv. Ther. 2018, 2, 1800091.
- Mehra, N.K.; Mishra, V.; Jain, N.K. Receptor-based targeting of therapeutics. Ther. Deliv. 2013, 4, 369–394.
- Grobmyer, S.R.; Zhou, G.; Gutwein, L.G.; Iwakuma, N.; Sharma, P.; Hochwald, S.N. Nanoparticle delivery for metastatic breast cancer. Maturitas 2012, 73, 19–26.
- Rizwanullah, M.; Ahmad, M.Z.; Garg, A.; Ahmad, J. Advancement in design of nanostructured lipid carriers for cancer targeting and theranostic application. Biochim. Biophys. Acta Gen. Subj. 2021, 1865, 129936.
- Vhora, I.; Patil, S.; Bhatt, P.; Gandhi, R.; Baradia, D.; Misra, A. Receptor-targeted drug delivery: Current perspective and challenges. Ther. Deliv. 2014, 5, 1007–1024.
- Morales-Cruz, M.; Delgado, Y.; Castillo, B.; Figueroa, C.M.; Molina, A.M.; Torres, A.; Milian, M.; Griebenow, K. Smart Targeting to Improve Cancer Therapeutics. Drug Des. Dev. Ther. 2019, ume 13, 3753–3772.
- Das, M.; Mohanty, C.; Sahoo, S.K. Ligand-based targeted therapy for cancer tissue. Expert Opin. Drug Deliv. 2009, 6, 285–304.
- Rosenblum, D.; Joshi, N.; Tao, W.; Karp, J.M.; Peer, D. Progress and challenges towards targeted delivery of cancer therapeutics. Nat. Commun. 2018, 9, 1410.
- Sigismund, S.; Avanzato, D.; Lanzetti, L. Emerging functions of the EGFR in cancer. Mol. Oncol. 2017, 12, 3–20.
- Pinkas-Kramarski, R.; Alroy, I.; Yarden, Y. ErbB receptors and EGF-like ligands: Cell lineage determination and oncogenesis through combinatorial signaling. J. Mammary Gland. Biol. Neoplasia 1997, 2, 97–107.
- Wee, P.; Wang, Z. Epidermal Growth Factor Receptor Cell Proliferation Signaling Pathways. Cancers 2017, 9, 52.
- Zhou, X.; Shi, K.; Hao, Y.; Yang, C.; Zha, R.; Yi, C.; Qian, Z. Advances in nanotechnology-based delivery systems for EGFR tyrosine kinases inhibitors in cancer therapy. Asian J. Pharm. Sci. 2019, 15, 26–41.
- Bossuyt, V.; Fadare, O.; Martel, M.; Ocal, I.T.; Burtness, B.; Moinfar, F.; Leibl, S.; Tavassoli, F.A. Remarkably High Frequency of EGFR Expression in Breast Carcinomas with Squamous Differentiation. Int. J. Surg. Pathol. 2005, 13, 319–327.
- Albanell, J.; Baselga, J. The ErbB receptors as targets for breast cancer therapy. J. Mammary Gland. Biol. Neoplasia 1999, 4, 337–351.
- Farasat, A.; Rahbarizadeh, F.; Ahmadvand, D.; Ranjbar, S.; Nikkhoi, S.K. Effective suppression of tumour cells by oligoclonal HER2-targeted delivery of liposomal doxorubicin. J. Liposome Res. 2018, 29, 53–65.
- Duan, D.; Wang, A.; Ni, L.; Zhang, L.; Yan, X.; Jiang, Y.; Mu, H.; Wu, Z.; Sun, K.; Li, Y. Trastuzumab- and Fab′ fragment-modified curcumin PEG-PLGA nanoparticles: Preparation and evaluation in vitro and in vivo. Int. J. Nanomed. 2018, ume 13, 1831–1840.
- Jin, H.; Pi, J.; Zhao, Y.; Jiang, J.; Li, T.; Zeng, X.; Yang, P.; Evans, C.E.; Cai, J. EGFR-targeting PLGA-PEG nanoparticles as a curcumin delivery system for breast cancer therapy. Nanoscale 2017, 9, 16365–16374.
- Zhao, J.; Feng, S.-S. Effects of PEG tethering chain length of vitamin E TPGS with a Herceptin-functionalized nanoparticle formulation for targeted delivery of anticancer drugs. Biomaterials 2014, 35, 3340–3347.
- Kutty, R.V.; Feng, S.-S. Cetuximab conjugated vitamin E TPGS micelles for targeted delivery of docetaxel for treatment of triple negative breast cancers. Biomaterials 2013, 34, 10160–10171.
- Milane, L.; Duan, Z.; Amiji, M. Development of EGFR-Targeted Polymer Blend Nanocarriers for Combination Paclitaxel/Lonidamine Delivery to Treat Multi-Drug Resistance in Human Breast and Ovarian Tumor Cells. Mol. Pharm. 2010, 8, 185–203.
- Dilnawaz, F.; Singh, A.; Mohanty, C.; Sahoo, S.K. Dual drug loaded superparamagnetic iron oxide nanoparticles for targeted cancer therapy. Biomaterials 2010, 31, 3694–3706.
- Acharya, S.; Dilnawaz, F.; Sahoo, S.K. Targeted epidermal growth factor receptor nanoparticle bioconjugates for breast cancer therapy. Biomaterials 2009, 30, 5737–5750.
- Sun, B.; Ranganathan, B.; Feng, S.-S. Multifunctional poly(d,l-lactide-co-glycolide)/montmorillonite (PLGA/MMT) nanoparticles decorated by Trastuzumab for targeted chemotherapy of breast cancer. Biomaterials 2008, 29, 475–486.
- Yu, B.; Tai, H.C.; Xue, W.; Lee, L.J.; Lee, R.J. Receptor-targeted nanocarriers for therapeutic delivery to cancer. Mol. Membr. Biol. 2010, 27, 286–298.
- Kedar, U.; Phutane, P.; Shidhaye, S.; Kadam, V. Advances in polymeric micelles for drug delivery and tumor targeting. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 714–729.
- Low, P.S.; Kularatne, S.A. Folate-targeted therapeutic and imaging agents for cancer. Curr. Opin. Chem. Biol. 2009, 13, 256–262.
- 102. Fernández, M.; Javaida, F.; Chudasama, V. Advances in targeting the folate receptor in the treatment/imaging of cancers. Chem. Sci. 2018, 9, 790–810.
- Ross, J.F.; Chaudhuri, P.K.; Ratnam, M. Differential regulation of folate receptor isoforms in normal and malignant tissues in vivo and in established cell lines. Physiologic and clinical implications. Cancer 1994, 73, 2432–2443.
- Cheung, A.; Bax, H.J.; Josephs, D.H.; Ilieva, K.M.; Pellizzari, G.; Opzoomer, J.; Bloomfield, J.; Fittall, M.; Grigoriadis, A.; Figini, M.; et al. Targeting folate receptor alpha for cancer treatment. Oncotarget 2016, 7, 52553–52574.
- Elnakat, H. Distribution, functionality and gene regulation of folate receptor isoforms: Implications in targeted therapy. Adv. Drug Deliv. Rev. 2004, 56, 1067–1084.
- Yingchoncharoen, P.; Kalinowski, D.S.; Richardson, D.R. Lipid-Based Drug Delivery Systems in Cancer Therapy: What Is Available and What Is Yet to Come. Pharmacol. Rev. 2016, 68, 701–787.
- Erdoğar, N.; Esendağlı, G.; Nielsen, T.T.; Esendağlı-Yılmaz, G.; Yoyen-Ermis, D.; Erdoğdu, B.; Sargon, M.F.; Eroğlu, H.; Bilensoy, E. Therapeutic efficacy of folate receptor-targeted amphiphilic cyclodextrin nanoparticles as a novel vehicle for paclitaxel delivery in breast cancer. J. Drug Target. 2017, 26, 66–74.
- Thapa, R.K.; Choi, J.Y.; Gupta, B.; Ramasamy, T.; Poudel, B.K.; Ku, S.K.; Youn, Y.S.; Choi, H.G.; Yong, C.S.; Kim, J.O. Liquid crystalline nanoparticles encapsulating cisplatin and docetaxel combination for targeted therapy of breast cancer. Biomater. Sci. 2016, 4, 1340–1350.
- Lin, M.; Teng, L.; Wang, Y.; Zhang, J.; Sun, X. Curcumin-guided nanotherapy: A lipid-based nanomedicine for targeted drug delivery in breast cancer therapy. Drug Deliv. 2015, 23, 1420–1425.
- Nguyen, D.H.; Lee, J.S.; Bae, J.W.; Choi, J.H.; Lee, Y.; Son, J.Y.; Park, K.D. Targeted doxorubicin nanotherapy strongly suppressing growth of multidrug resistant tumor in mice. Int. J. Pharm. 2015, 495, 329–335.
- Gunduz, U.; Keskin, T.; Tansık, G.; Mutlu, P.; Yalcın, S.; Unsoy, G.; Yakar, A.; Khodadust, R.; Gunduz, G. Idarubicin-loaded folic acid conjugated magnetic nanoparticles as a targetable drug delivery system for breast cancer. Biomed. Pharmacother. 2014, 68, 729–736.
- Zhang, D.; Wang, F.; Chen, Y.; Zhang, Q.; Zheng, D.; Hao, L.; Liu, Y.; Duan, C.; Jia, L.; Liu, G. Folate-mediated targeted and intracellular delivery of paclitaxel using a novel deoxycholic acid-O-carboxymethylated chitosan–folic acid micelles. Int. J. Nanomed. 2012, 7, 325–337.
- Chen, J.; Li, S.; Shen, Q.; He, H.; Zhang, Y. Enhanced cellular uptake of folic acid–conjugated PLGA–PEG nanoparticles loaded with vincristine sulfate in human breast cancer. Drug Dev. Ind. Pharm. 2011, 37, 1339–1346.
- Osborne, C.K. Steroid hormone receptors in breast cancer management. Breast Cancer Res. Treat. 1998, 51, 227–238.
- Rai, S.; Paliwal, R.; Vaidya, B.; Khatri, K.; Goyal, A.K.; Gupta, P.N.; Vyas, S.P. Targeted delivery of doxorubicin via estrone-appended liposomes. J. Drug Target. 2008, 16, 455–463.
- Lumachi, F.; Santeufemia, D.; Basso, S.M.M. Current medical treatment of estrogen receptor-positive breast cancer. World J. Biol. Chem. 2015, 6, 231–239.
- Tang, H.; Chen, J.; Wang, L.; Li, Q.; Yang, Y.; Lv, Z.; Bao, H.; Li, Y.; Luan, X.; Li, Y.; et al. Co-delivery of epirubicin and paclitaxel using an estrone-targeted PEGylated liposomal nanoparticle for breast cancer. Int. J. Pharm. 2019, 573, 118806.
- Jain, A.S.; Goel, P.N.; Shah, S.M.; Dhawan, V.V.; Nikam, Y.; Gude, R.P.; Nagarsenker, M.S. Tamoxifen guided liposomes for targeting encapsulated anticancer agent to estrogen receptor positive breast cancer cells: In vitro and in vivo evaluation. Biomed. Pharmacother. 2014, 68, 429–438.
- Paliwal, S.R.; Mishra, N.; Mehta, A.; Vyas, S. A Novel Cancer Targeting Approach Based on Estrone Anchored Stealth Liposome for Site-Specific Breast Cancer Therapy. Curr. Cancer Drug Targets 2010, 10, 343–353.
- Dreaden, E.; Mwakwari, S.C.; Sodji, Q.H.; Oyelere, A.K.; El-Sayed, M.A. Tamoxifen−Poly(ethylene glycol)−Thiol Gold Nanoparticle Conjugates: Enhanced Potency and Selective Delivery for Breast Cancer Treatment. Bioconjugate Chem. 2009, 20, 2247–2253.
- Zöller, M. CD44: Physiological expression of distinct isoforms as evidence for organ-specific metastasis formation. J. Mol. Med. 1995, 73, 425–438.
- Ohene-Abuakwa, Y.; Pignatelli, M. Adhesion Molecules in Cancer Biology. Adv. Exp. Med. Biol. 2002, 465, 115–126.
- Fares, J.; Fares, M.Y.; Khachfe, H.H.; Salhab, H.A.; Fares, Y. Molecular principles of metastasis: A hallmark of cancer revisited. Signal. Transduct. Target. Ther. 2020, 5, 28.
- Gallatin, M.; John, T.P.S.; Siegelman, M.; Reichert, R.; Butcher, E.C.; Weissman, I.L. Lymphocyte homing receptors. Cell 1986, 44, 673–680.
- Nemec, R.E.; Toole, B.P.; Knudson, W. The cell surface hyaluronate binding sites of invasive human bladder carcinoma cells. Biochem. Biophys. Res. Commun. 1987, 149, 249–257.
- Bartolazzi, A.; Peach, R.; Aruffo, A.; Stamenkovic, I. Interaction between CD44 and hyaluronate is directly implicated in the regulation of tumor development. J. Exp. Med. 1994, 180, 53–66.
- Patrawala, L.; Calhoun, T.; Schneiderbroussard, R.; Li, H.; Bhatia, B.; Tang, S.; Reilly, J.; Chandra, D.; Zhou, J.; Claypool, K.; et al. Highly purified CD44+ prostate cancer cells from xenograft human tumors are enriched in tumorigenic and metastatic progenitor cells. Oncogene 2006, 25, 1696–1708.
- Prince, M.E.; Sivanandan, R.; Kaczorowski, A.; Wolf, G.T.; Kaplan, M.J.; Dalerba, P.; Weissman, I.L.; Clarke, M.F.; Ailles, L.E. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc. Natl. Acad. Sci. USA 2007, 104, 973–978.
- Surace, C.; Arpicco, S.; Dufaÿ-Wojcicki, A.; Marsaud, V.; Bouclier, C.; Clay, D.; Cattel, L.; Renoir, J.-M.; Fattal, E. Lipoplexes Targeting the CD44 Hyaluronic Acid Receptor for Efficient Transfection of Breast Cancer Cells. Mol. Pharm. 2009, 6, 1062–1073.
- Palomeras, S.; Ruiz-Martínez, S.; Puig, T. Targeting Breast Cancer Stem Cells to Overcome Treatment Resistance. Molecules 2018, 23, 2193.
- Rezaei, S.; Kashanian, S.; Bahrami, Y.; Cruz, L.J.; Motiei, M. Redox-Sensitive and Hyaluronic Acid-Functionalized Nanoparticles for Improving Breast Cancer Treatment by Cytoplasmic 17α-Methyltestosterone Delivery. Molecules 2020, 25, 1181.
- Cerqueira, B.B.S.; Lasham, A.; Shelling, A.N.; Al-Kassas, R. Development of biodegradable PLGA nanoparticles surface engineered with hyaluronic acid for targeted delivery of paclitaxel to triple negative breast cancer cells. Mater. Sci. Eng. C 2017, 76, 593–600.
- Liu, Q.; Li, J.; Pu, G.; Zhang, F.; Liu, H.; Zhang, Y. Co-delivery of baicalein and doxorubicin by hyaluronic acid decorated nanostructured lipid carriers for breast cancer therapy. Drug Deliv. 2015, 23, 1364–1368.
- Zhong, Y.; Zhang, J.; Cheng, R.; Deng, C.; Meng, F.; Xie, F.; Zhong, Z. Reversibly crosslinked hyaluronic acid nanoparticles for active targeting and intelligent delivery of doxorubicin to drug resistant CD44+ human breast tumor xenografts. J. Control. Release 2015, 205, 144–154.
- Zhao, Y.; Zhang, T.; Duan, S.; Davies, N.M.; Forrest, M.L. CD44-tropic polymeric nanocarrier for breast cancer targeted rapamycin chemotherapy. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 1221–1230.
- Huang, J.; Zhang, H.; Yu, Y.; Chen, Y.; Wang, D.; Zhang, G.; Zhou, G.; Liu, J.; Sun, Z.; Sun, D.; et al. Biodegradable self-assembled nanoparticles of poly (d,l-lactide-co-glycolide)/hyaluronic acid block copolymers for target delivery of docetaxel to breast cancer. Biomaterials 2013, 35, 550–566.
- Yang, C.; Liu, Y.; He, Y.; Du, Y.; Wang, W.; Shi, X.; Gao, F. The use of HA oligosaccharide-loaded nanoparticles to breach the endogenous hyaluronan glycocalyx for breast cancer therapy. Biomaterials 2013, 34, 6829–6838.
- Li, X.; Taratula, O.; Taratula, O.; Schumann, C.; Minko, T. LHRH-Targeted Drug Delivery Systems for Cancer Therapy. Mini-Rev. Med. Chem. 2017, 17, 258–267.
- Ghanghoria, R.; Kesharwani, P.; Tekade, R.K.; Jain, N.K. Targeting luteinizing hormone-releasing hormone: A potential therapeutics to treat gynecological and other cancers. J. Control. Release 2018, 269, 277–301.
- Obayemi, J.D.; Salifu, A.A.; Eluu, S.C.; Uzonwanne, V.O.; Jusu, S.M.; Nwazojie, C.C.; Onyekanne, C.E.; Ojelabi, O.; Payne, L.; Moore, C.M.; et al. LHRH-Conjugated Drugs as Targeted Therapeutic Agents for the Specific Targeting and Localized Treatment of Triple Negative Breast Cancer. Sci. Rep. 2020, 10, 1–18.
- Hu, J.; Obayemi, J.; Malatesta, K.; Košmrlj, A.; Soboyejo, W. Enhanced cellular uptake of LHRH-conjugated PEG-coated magnetite nanoparticles for specific targeting of triple negative breast cancer cells. Mater. Sci. Eng. C 2018, 88, 32–45.
- Varshosaz, J.; Hassanzadeh, F.; Aliabadi, H.S.; Khoraskani, F.R.; Mirian, M.; Behdadfar, B. Targeted delivery of doxorubicin to breast cancer cells by magnetic LHRH chitosan bioconjugated nanoparticles. Int. J. Biol. Macromol. 2016, 93, 1192–1205.
- Li, M.; Tang, Z.; Zhang, Y.; Lv, S.; Li, Q.; Chen, X. Targeted delivery of cisplatin by LHRH-peptide conjugated dextran nanoparticles suppresses breast cancer growth and metastasis. Acta Biomater. 2015, 18, 132–143.
- Taheri, A.; Dinarvand, R.; Ahadi, F.; Khorramizadeh, M.R.; Atyabi, F. The in vivo antitumor activity of LHRH targeted methotrexate–human serum albumin nanoparticles in 4T1 tumor-bearing Balb/c mice. Int. J. Pharm. 2012, 431, 183–189.
More
