In the automotive field the term “particulate matter (PM)” is used for the collected matter on a flow-through filter under specific conditions, and the term “particle” for aerosol particles measured while airborne (suspended matter). Particles are divided into “volatile” and “non-volatile” (or solid) at tailpipe conditions (high temperature, high concentration). Species that at tailpipe conditions appear volatile, may partition toward the particulate phase at atmospheric conditions (low temperature), and the term semi-volatile better characterizes them [41]. The term “semi-volatiles” (instead of “volatiles”) will be used loosely in this text to indicate species not counted after dilution and thermal pre-treatment at 300–400 °C.
The term ultrafine particles (i.e., particles < 100 nm) is not so common in the automotive community. Even though the majority of particles has sizes <100 nm, the tail extends to larger sizes. A recent review argued that a better definition for ultrafine particles (focusing on the automotive field) would be particles <500 nm [16].
- primary aerosol
- fresh aerosol
- secondary aerosol
- nucleation mode
- vehicle emissions
- road transport
- urban pollution
- air quality
- PMP
- PEMS
1. Introduction
2. PM Emitted from Vehicles
2.1. Primary “Tailpipe” Particles
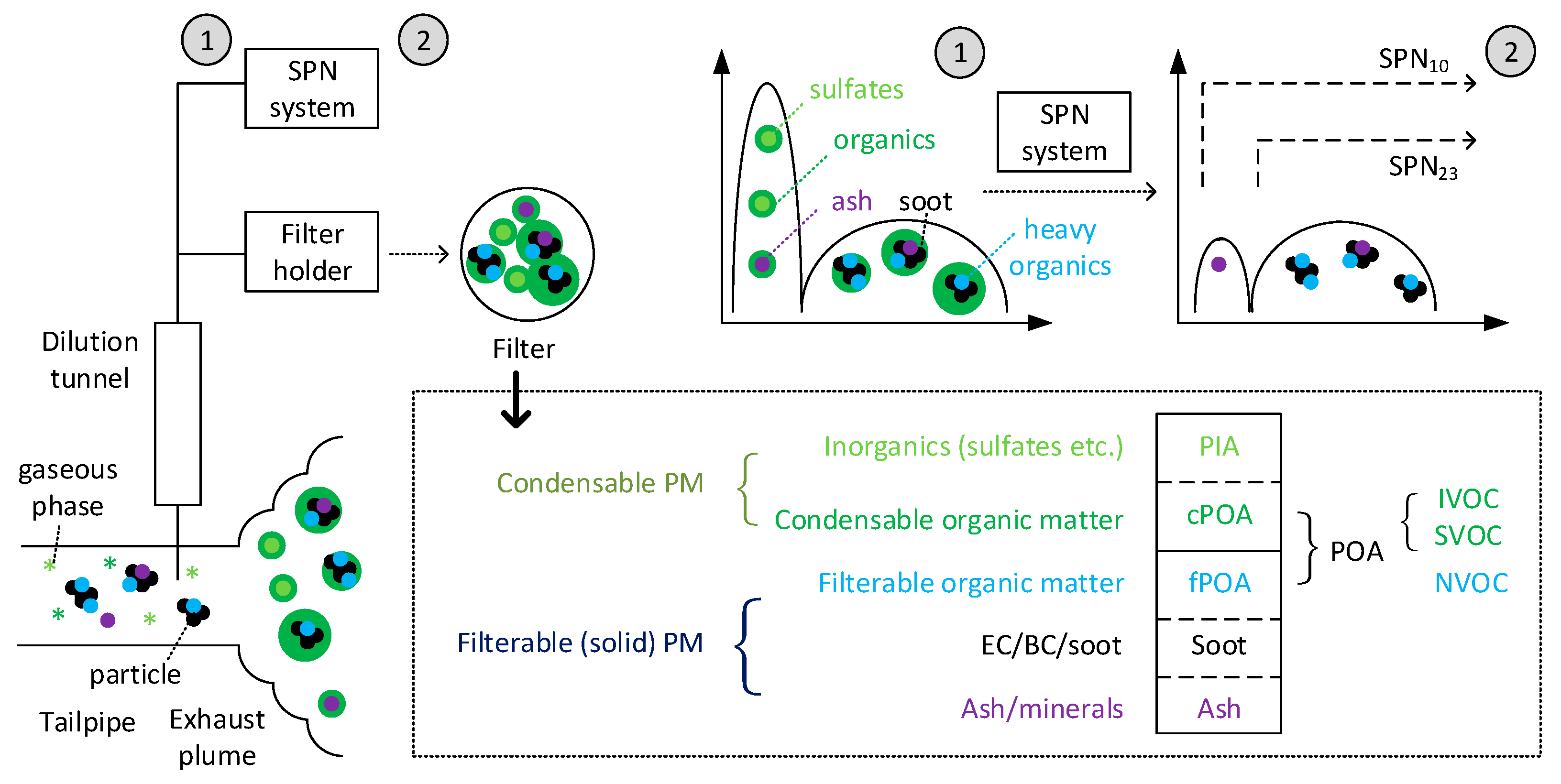
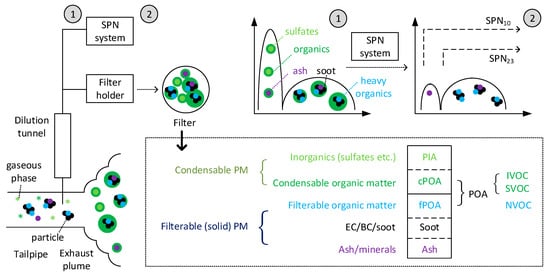
2.2. Delayed Primary “Fresh” Particles
2.3. Secondary Particles
2.4. Emission Levels of Solid and Volatile Particles
References
- World Health Organization. WHO Global Air Quality Guidelines: Particulate Matter (PM2.5 and PM10), Ozone, Nitrogen Dioxide, Sulfur Dioxide and Carbon Monoxide; World Health Organization: Geneva, Switzerland, 2021; ISBN 978-92-4-003422-8.
- Wolf, K.; Hoffmann, B.; Andersen, Z.J.; Atkinson, R.W.; Bauwelinck, M.; Bellander, T.; Brandt, J.; Brunekreef, B.; Cesaroni, G.; Chen, J.; et al. Long-term exposure to low-level ambient air pollution and incidence of stroke and coronary heart disease: A pooled analysis of six European cohorts within the ELAPSE project. Lancet Planet. Health 2021, 5, e620–e632.
- Manisalidis, I.; Stavropoulou, E.; Stavropoulos, A.; Bezirtzoglou, E. Environmental and Health Impacts of Air Pollution: A Review. Front. Public Health 2020, 8, 14.
- Hamanaka, R.B.; Mutlu, G.M. Particulate Matter Air Pollution: Effects on the Cardiovascular System. Front. Endocrinol. 2018, 9, 680.
- European Environment Agency. Air Quality in Europe: 2020 Report; Publications Office of the European Union: Luxembourg, 2020.
- Zhang, R.; Wang, G.; Guo, S.; Zamora, M.L.; Ying, Q.; Lin, Y.; Wang, W.; Hu, M.; Wang, Y. Formation of Urban Fine Particulate Matter. Chem. Rev. 2015, 115, 3803–3855.
- Giechaskiel, B.; Mamakos, A.; Andersson, J.; Dilara, P.; Martini, G.; Schindler, W.; Bergmann, A. Measurement of Automotive Nonvolatile Particle Number Emissions within the European Legislative Framework: A Review. Aerosol Sci. Technol. 2012, 46, 719–749.
- Wang, J.M.; Jeong, C.-H.; Zimmerman, N.; Healy, R.M.; Hilker, N.; Evans, G.J. Real-World Emission of Particles from Vehicles: Volatility and the Effects of Ambient Temperature. Environ. Sci. Technol. 2017, 51, 4081–4090.
- ICCT. ICCT’s Comments and Technical Recommendations on Future Euro 7/VII Emission Standards; International Council on Clean Transportation: Berlin, Germany, 2021.
- Simonen, P.; Kalliokoski, J.; Karjalainen, P.; Rönkkö, T.; Timonen, H.; Saarikoski, S.; Aurela, M.; Bloss, M.; Triantafyllopoulos, G.; Kontses, A.; et al. Characterization of laboratory and real driving emissions of individual Euro 6 light-duty vehicles—Fresh particles and secondary aerosol formation. Environ. Pollut. 2019, 255, 113175.
- Rönkkö, T.; Timonen, H. Overview of Sources and Characteristics of Nanoparticles in Urban Traffic-Influenced Areas. J. Alzheimer’s Dis. 2019, 72, 15–28.
- Kittelson, D.; Khalek, I.; McDonald, J.; Stevens, J.; Giannelli, R. Particle emissions from mobile sources: Discussion of ultrafine particle emissions and definition. J. Aerosol Sci. 2021, 159, 105881.
- Simpson, D.; Fagerli, H.; Colette, A.; van der Gon, H.D.; Dore, C.; Hallquist, M.; Hansson, H.C.; Maas, R.; Rouil, L.; Allemand, N.; et al. How Should Condensables Be Included in PM Emission Inventories Reported to EMEP/CLRTAP? EMEP: Gothenburg, Sweden, 2020.
- Giechaskiel, B.; Maricq, M.; Ntziachristos, L.; Dardiotis, C.; Wang, X.; Axmann, H.; Bergmann, A.; Schindler, W. Review of motor vehicle particulate emissions sampling and measurement: From smoke and filter mass to particle number. J. Aerosol Sci. 2014, 67, 48–86.
- Harris, S.J.; Maricq, M. Signature size distributions for diesel and gasoline engine exhaust particulate matter. J. Aerosol Sci. 2001, 32, 749–764.
- Giechaskiel, B.; Lähde, T.; Gandi, S.; Keller, S.; Kreutziger, P.; Mamakos, A. Assessment of 10-nm Particle Number (PN) Portable Emissions Measurement Systems (PEMS) for Future Regulations. Int. J. Environ. Res. Public Health 2020, 17, 3878.
- Khalek, I.A.; Badshah, H.; Premnath, V.; Brezny, R. Solid Particle Number and Ash Emissions from Heavy-Duty Natural Gas and Diesel w/SCRF Engines; SAE International: Warrendale, PA, USA, 2018.
- Wang, B.; Lau, Y.-S.; Huang, Y.; Organ, B.; Chuang, H.-C.; Ho, S.S.H.; Qu, L.; Lee, S.-C.; Ho, K.-F. Chemical and toxicological characterization of particulate emissions from diesel vehicles. J. Hazard. Mater. 2020, 405, 124613.
- Xing, J.; Shao, L.; Zhang, W.; Peng, J.; Wang, W.; Hou, C.; Shuai, S.; Hu, M.; Zhang, D. Morphology and composition of particles emitted from a port fuel injection gasoline vehicle under real-world driving test cycles. J. Environ. Sci. 2018, 76, 339–348.
- Polidori, A.; Hu, S.; Biswas, S.; Delfino, R.J.; Sioutas, C. Real-time characterization of particle-bound polycyclic aromatic hydrocarbons in ambient aerosols and from motor-vehicle exhaust. Atmos. Chem. Phys. 2008, 8, 1277–1291.
- Dutcher, D.D.; Stolzenburg, M.R.; Thompson, S.L.; Medrano, J.M.; Gross, D.S.; Kittelson, D.B.; McMurry, P.H. Emissions from Ethanol-Gasoline Blends: A Single Particle Perspective. Atmosphere 2011, 2, 182–200.
- Perrone, M.G.; Carbone, C.; Faedo, D.; Ferrero, L.; Maggioni, A.; Sangiorgi, G.; Bolzacchini, E. Exhaust emissions of polycyclic aromatic hydrocarbons, n-alkanes and phenols from vehicles coming within different European classes. Atmos. Environ. 2014, 82, 391–400.
- Muñoz, M.; Heeb, N.V.; Haag, R.; Honegger, P.; Zeyer, K.; Mohn, J.; Comte, P.; Czerwinski, J. Bioethanol Blending Reduces Nanoparticle, PAH, and Alkyl- and Nitro-PAH Emissions and the Genotoxic Potential of Exhaust from a Gasoline Direct Injection Flex-Fuel Vehicle. Environ. Sci. Technol. 2016, 50, 11853–11861.
- Cao, X.; Hao, X.; Shen, X.; Jiang, X.; Wu, B.; Yao, Z. Emission characteristics of polycyclic aromatic hydrocarbons and nitro-polycyclic aromatic hydrocarbons from diesel trucks based on on-road measurements. Atmos. Environ. 2017, 148, 190–196.
- Kwon, S.-B.; Lee, K.W.; Saito, K.; Shinozaki, O.; Seto, T. Size-Dependent Volatility of Diesel Nanoparticles: Chassis Dynamometer Experiments. Environ. Sci. Technol. 2003, 37, 1794–1802.
- Rönkkö, T.; Virtanen, A.; Kannosto, J.; Keskinen, J.; Lappi, M.; Pirjola, L. Nucleation Mode Particles with a Nonvolatile Core in the Exhaust of a Heavy Duty Diesel Vehicle. Environ. Sci. Technol. 2007, 41, 6384–6389.
- De Filippo, A.; Maricq, M.M. Diesel Nucleation Mode Particles: Semivolatile or Solid? Environ. Sci. Technol. 2008, 42, 7957–7962.
- Kirchner, U.; Scheer, V.; Vogt, R.; Kägi, R. TEM study on volatility and potential presence of solid cores in nucleation mode particles from diesel powered passenger cars. J. Aerosol Sci. 2009, 40, 55–64.
- Mayer, A.; Czerwinski, J.; Kasper, M.; Ulrich, A.; Mooney, J.J. Metal Oxide Particle Emissions from Diesel and Petrol Engines; SAE International: Warrendale, PA, USA, 2012.
- Sgro, L.A.; Sementa, P.; Vaglieco, B.M.; Rusciano, G.; D’Anna, A.; Minutolo, P. Investigating the origin of nuclei particles in GDI engine exhausts. Combust. Flame 2012, 159, 1687–1692.
- Liati, A.; Schreiber, D.; Dasilva, Y.A.R.; Eggenschwiler, P.D. Ultrafine particle emissions from modern Gasoline and Diesel vehicles: An electron microscopic perspective. Environ. Pollut. 2018, 239, 661–669.
- Seong, H.; Choi, S.; Lee, K. Examination of nanoparticles from gasoline direct-injection (GDI) engines using transmission electron microscopy (TEM). Int. J. Automot. Technol. 2014, 15, 175–181.
- Fushimi, A.; Kondo, Y.; Kobayashi, S.; Fujitani, Y.; Saitoh, K.; Takami, A.; Tanabe, K. Chemical composition and source of fine and nanoparticles from recent direct injection gasoline passenger cars: Effects of fuel and ambient temperature. Atmos. Environ. 2016, 124, 77–84.
- Kuuluvainen, H.; Karjalainen, P.; Saukko, E.; Ovaska, T.; Sirviö, K.; Honkanen, M.; Olin, M.; Niemi, S.; Keskinen, J.; Rönkkö, T. Nonvolatile ultrafine particles observed to form trimodal size distributions in non-road diesel engine exhaust. Aerosol Sci. Technol. 2020, 54, 1345–1358.
- Mamakos, A.; Schwelberger, M.; Fierz, M.; Giechaskiel, B. Effect of selective catalytic reduction on exhaust nonvolatile particle emissions of Euro VI heavy-duty compression ignition vehicles. Aerosol Sci. Technol. 2019, 53, 898–910.
- Giechaskiel, B. Solid Particle Number Emission Factors of Euro VI Heavy-Duty Vehicles on the Road and in the Laboratory. Int. J. Environ. Res. Public Health 2018, 15, 304.
- Rönkkö, T.; Kuuluvainen, H.; Karjalainen, P.; Keskinen, J.; Hillamo, R.; Niemi, J.; Pirjola, L.; Timonen, H.J.; Saarikoski, S.; Saukko, E.; et al. Traffic is a major source of atmospheric nanocluster aerosol. Proc. Natl. Acad. Sci. USA 2017, 114, 7549–7554.
- Alanen, J.; Saukko, E.; Lehtoranta, K.; Murtonen, T.; Timonen, H.; Hillamo, R.; Karjalainen, P.; Kuuluvainen, H.; Harra, J.; Keskinen, J.; et al. The formation and physical properties of the particle emissions from a natural gas engine. Fuel 2015, 162, 155–161.
- Maricq, M.M. Chemical characterization of particulate emissions from diesel engines: A review. J. Aerosol Sci. 2007, 38, 1079–1118.
- Karjalainen, P.; Pirjola, L.; Heikkilä, J.; Lähde, T.; Tzamkiozis, T.; Ntziachristos, L.; Keskinen, J.; Rönkkö, T. Exhaust particles of modern gasoline vehicles: A laboratory and an on-road study. Atmos. Environ. 2014, 97, 262–270.
- Ma, C.; Wu, L.; Mao, H.-J.; Fang, X.-Z.; Wei, N.; Zhang, J.-S.; Yang, Z.-W.; Zhang, Y.-J.; Lv, Z.-Y.; Yang, L. Transient Characterization of Automotive Exhaust Emission from Different Vehicle Types Based on On-Road Measurements. Atmosphere 2020, 11, 64.
- Rönkkö, T.; Pirjola, L.; Ntziachristos, L.; Heikkilä, J.; Karjalainen, P.; Hillamo, R.; Keskinen, J. Vehicle Engines Produce Exhaust Nanoparticles Even When Not Fueled. Environ. Sci. Technol. 2014, 48, 2043–2050.
- Sirignano, M.; D’Anna, A. Filtration and coagulation efficiency of sub-10 nm combustion-generated particles. Fuel 2018, 221, 298–302.
- Gren, L.; Malmborg, V.B.; Falk, J.; Markula, L.; Novakovic, M.; Shamun, S.; Eriksson, A.C.; Kristensen, T.B.; Svenningsson, B.; Tunér, M.; et al. Effects of renewable fuel and exhaust aftertreatment on primary and secondary emissions from a modern heavy-duty diesel engine. J. Aerosol Sci. 2021, 156, 105781.
- Liati, A.; Spiteri, A.; Eggenschwiler, P.D.; Vogel-Schäuble, N. Microscopic investigation of soot and ash particulate matter derived from biofuel and diesel: Implications for the reactivity of soot. J. Nanopart. Res. 2012, 14, 1224.
- Muñoz, M.; Haag, R.; Zeyer, K.; Mohn, J.; Comte, P.; Czerwinski, J.; Heeb, N.V. Effects of Four Prototype Gasoline Particle Filters (GPFs) on Nanoparticle and Genotoxic PAH Emissions of a Gasoline Direct Injection (GDI) Vehicle. Environ. Sci. Technol. 2018, 52, 10709–10718.
- Kittelson, D.B. Engines and nanoparticles: A review. J. Aerosol Sci. 1998, 29, 575–588.
- Uy, D.; Storey, J.; Sluder, C.S.; Barone, T.; Lewis, S.; Jagner, M. Effects of Oil Formulation, Oil Separator, and Engine Speed and Load on the Particle Size, Chemistry, and Morphology of Diesel Crankcase Aerosols. SAE Int. J. Fuels Lubr. 2016, 9, 224–238.
- Giechaskiel, B.; Ntziachristos, L.; Samaras, Z.; Casati, R.; Scheer, V.; Vogt, R. Effect of Speed and Speed-Transition on the Formation of Nucleation Mode Particles from a Light Duty Diesel Vehicle; SAE International: Warrendale, PA, USA, 2007.
- Suarez-Bertoa, R.; Mendoza-Villafuerte, P.; Riccobono, F.; Vojtisek, M.; Pechout, M.; Perujo, A.; Astorga, C. On-road measurement of NH3 emissions from gasoline and diesel passenger cars during real world driving conditions. Atmos. Environ. 2017, 166, 488–497.
- Selleri, T.; Melas, A.; Joshi, A.; Manara, D.; Perujo, A.; Suarez-Bertoa, R. An Overview of Lean Exhaust deNOx Aftertreatment Technologies and NOx Emission Regulations in the European Union. Catalysts 2021, 11, 404.
- Charron, A.; Harrison, R.M. Primary particle formation from vehicle emissions during exhaust dilution in the roadside atmosphere. Atmos. Environ. 2003, 37, 4109–4119.
- Casati, R.; Scheer, V.; Vogt, R.; Benter, T. Measurement of nucleation and soot mode particle emission from a diesel passenger car in real world and laboratory in situ dilution. Atmos. Environ. 2007, 41, 2125–2135.
- Uhrner, U.; Zallinger, M.; von Löwis, S.; Vehkamäki, H.; Wehner, B.; Stratmann, F.; Wiedensohler, A. Volatile Nanoparticle Formation and Growth within a Diluting Diesel Car Exhaust. J. Air Waste Manag. Assoc. 2011, 61, 399–408.
- Tsai, J.-H.; Chang, S.-Y.; Chiang, H.-L. Volatile organic compounds from the exhaust of light-duty diesel vehicles. Atmos. Environ. 2012, 61, 499–506.
- Rönkkö, T.; Lähde, T.; Heikkilä, J.; Pirjola, L.; Bauschke, U.; Arnold, F.; Schlager, H.; Rothe, D.; Yli-Ojanperä, J.; Keskinen, J. Effects of Gaseous Sulphuric Acid on Diesel Exhaust Nanoparticle Formation and Characteristics. Environ. Sci. Technol. 2013, 47, 11882–11889.
- Kittelson, D.; Watts, W.; Johnson, J.; Rowntree, C.; Payne, M.; Goodier, S.; Warrens, C.; Preston, H.; Zink, U.; Ortiz, M.; et al. On-road evaluation of two Diesel exhaust aftertreatment devices. J. Aerosol Sci. 2006, 37, 1140–1151.
- Rodríguez, S.; Cuevas, E. The contributions of “minimum primary emissions” and “new particle formation enhancements” to the particle number concentration in urban air. J. Aerosol Sci. 2007, 38, 1207–1219.
- Zhou, L.; Hallquist, Å.M.; Hallquist, M.; Salvador, C.M.; Gaita, S.M.; Sjödin, Å.; Jerksjö, M.; Salberg, H.; Wängberg, I.; Mellqvist, J.; et al. A transition of atmospheric emissions of particles and gases from on-road heavy-duty trucks. Atmos. Chem. Phys. 2020, 20, 1701–1722.
- Vaaraslahti, K.; Virtanen, A.; Ristimäki, J.; Keskinen, J. Nucleation Mode Formation in Heavy-Duty Diesel Exhaust with and without a Particulate Filter. Environ. Sci. Technol. 2004, 38, 4884–4890.
- Giechaskiel, B.; Ntziachristos, L.; Samaras, Z.; Scheer, V.; Casati, R.; Vogt, R. Formation potential of vehicle exhaust nucleation mode particles on-road and in the laboratory. Atmos. Environ. 2005, 39, 3191–3198.
- Vaaraslahti, K.; Keskinen, J.; Giechaskiel, B.; Solla, A.; Murtonen, T.; Vesala, H. Effect of Lubricant on the Formation of Heavy-Duty Diesel Exhaust Nanoparticles. Environ. Sci. Technol. 2005, 39, 8497–8504.
- Vouitsis, E.; Ntziachristos, L.; Samaras, Z. Modelling of diesel exhaust aerosol during laboratory sampling. Atmos. Environ. 2005, 39, 1335–1345.
- Du, H.; Yu, F. Nanoparticle formation in the exhaust of vehicles running on ultra-low sulfur fuel. Atmos. Chem. Phys. Discuss. 2008, 8, 4729–4739.
- Lemmetty, M.; Rönkkö, T.; Virtanen, A.; Keskinen, J.; Pirjola, L. The Effect of Sulphur in Diesel Exhaust Aerosol: Models Compared with Measurements. Aerosol Sci. Technol. 2008, 42, 916–929.
- Olin, M.; Rönkkö, T.; Maso, M.D. CFD modeling of a vehicle exhaust laboratory sampling system: Sulfur-driven nucleation and growth in diluting diesel exhaust. Atmos. Chem. Phys. Discuss. 2015, 15, 5305–5323.
- Sakurai, H.; Tobias, H.; Park, K.; Zarling, D.; Docherty, K.S.; Kittelson, D.B.; McMurry, P.H.; Ziemann, P.J. On-line measurements of diesel nanoparticle composition and volatility. Atmos. Environ. 2003, 37, 1199–1210.
- Park, G.; Mun, S.; Hong, H.; Chung, T.; Jung, S.; Kim, S.; Seo, S.; Kim, J.; Lee, J.; Kim, K.; et al. Characterization of Emission Factors Concerning Gasoline, LPG, and Diesel Vehicles via Transient Chassis-Dynamometer Tests. Appl. Sci. 2019, 9, 1573.
- Ristimäki, J.; Lehtoranta, K.; Lappi, M.; Keskinen, J. Hydrocarbon Condensation in Heavy-Duty Diesel Exhaust. Environ. Sci. Technol. 2007, 41, 6397–6402.
- Pirjola, L.; Karjalainen, P.; Heikkilä, J.; Saari, S.; Tzamkiozis, T.; Ntziachristos, L.; Kulmala, K.; Keskinen, J.; Rönkkö, T. Effects of Fresh Lubricant Oils on Particle Emissions Emitted by a Modern Gasoline Direct Injection Passenger Car. Environ. Sci. Technol. 2015, 49, 3644–3652.
- Vogt, R.; Scheer, V.; Casati, R.; Benter, T. On-Road Measurement of Particle Emission in the Exhaust Plume of a Diesel Passenger Car. Environ. Sci. Technol. 2003, 37, 4070–4076.
- Kostenidou, E.; Martinez-Valiente, A.; R’Mili, B.; Marques, B.; Temime-Roussel, B.; Durand, A.; André, M.; Liu, Y.; Louis, C.; Vansevenant, B.; et al. Technical note: Emission factors, chemical composition, and morphology of particles emitted from Euro 5 diesel and gasoline light-duty vehicles during transient cycles. Atmos. Chem. Phys. 2021, 21, 4779–4796.
- Bergmann, M.; Kirchner, U.; Vogt, R.; Benter, T. On-road and laboratory investigation of low-level PM emissions of a modern diesel particulate filter equipped diesel passenger car. Atmos. Environ. 2009, 43, 1908–1916.
- Tzamkiozis, T.; Ntziachristos, L.; Samaras, Z. Diesel passenger car PM emissions: From Euro 1 to Euro 4 with particle filter. Atmos. Environ. 2010, 44, 909–916.
- Keskinen, J.; Rönkkö, T. Can Real-World Diesel Exhaust Particle Size Distribution be Reproduced in the Laboratory? A Critical Review Jorma Keskinen. J. Air Waste Manag. Assoc. 2010, 60, 1245–1255.
- Lu, T.; Cheung, C.S.; Huang, Z. Size-Resolved Volatility, Morphology, Nanostructure, and Oxidation Characteristics of Diesel Particulate. Energy Fuels 2012, 26, 6168–6176.
- Pang, Y.; Fuentes, M.; Rieger, P. Trends in the emissions of Volatile Organic Compounds (VOCs) from light-duty gasoline vehicles tested on chassis dynamometers in Southern California. Atmos. Environ. 2013, 83, 127–135.
- Yang, J.; Roth, P.; Zhu, H.; Durbin, T.D.; Karavalakis, G. Impacts of gasoline aromatic and ethanol levels on the emissions from GDI vehicles: Part 2. Influence on particulate matter, black carbon, and nanoparticle emissions. Fuel 2019, 252, 812–820.
- Clairotte, M.; Adam, T.; Zardini, A.; Manfredi, U.; Martini, G.; Krasenbrink, A.; Vicet, A.; Tournié, E.; Astorga, C. Effects of low temperature on the cold start gaseous emissions from light duty vehicles fuelled by ethanol-blended gasoline. Appl. Energy 2013, 102, 44–54.
- Sonntag, D.B.; Bailey, C.R.; Fulper, C.R.; Baldauf, R.W. Contribution of Lubricating Oil to Particulate Matter Emissions from Light-Duty Gasoline Vehicles in Kansas City. Environ. Sci. Technol. 2012, 46, 4191–4199.
- Amirante, R.; Distaso, E.; Napolitano, M.; Tamburrano, P.; Di Iorio, S.; Sementa, P.; Vaglieco, B.M.; Reitz, R.D. Effects of lubricant oil on particulate emissions from port-fuel and direct-injection spark-ignition engines. Int. J. Engine Res. 2017, 18, 606–620.
- Distaso, E.; Amirante, R.; Calò, G.; De Palma, P.; Tamburrano, P. Evolution of Soot Particle Number, Mass and Size Distribution along the Exhaust Line of a Heavy-Duty Engine Fueled with Compressed Natural Gas. Energies 2020, 13, 3993.
- Karavalakis, G.; Durbin, T.D.; Yang, J.; Ventura, L.; Xu, K. Fuel Effects on PM Emissions from Different Vehicle/Engine Configurations: A Literature Review; SAE International: Warrendale, PA, USA, 2018.
- Lu, Q.; Zhao, Y.; Robinson, A.L. Comprehensive organic emission profiles for gasoline, diesel, and gas-turbine engines including intermediate and semi-volatile organic compound emissions. Atmos. Chem. Phys. 2018, 18, 17637–17654.
- Cheung, K.L.; Polidori, A.; Ntziachristos, L.; Tzamkiozis, T.; Samaras, Z.; Cassee, F.R.; Gerlofs, M.; Sioutas, C. Chemical Characteristics and Oxidative Potential of Particulate Matter Emissions from Gasoline, Diesel, and Biodiesel Cars. Environ. Sci. Technol. 2009, 43, 6334–6340.
- Giechaskiel, B.; Melas, A.D.; Lähde, T.; Martini, G. Non-Volatile Particle Number Emission Measurements with Catalytic Strippers: A Review. Vehicles 2020, 2, 342–364.
- Khalek, I.A.; Bougher, T.L.; Merritt, P.M.; Zielinska, B. Regulated and Unregulated Emissions from Highway Heavy-Duty Diesel Engines Complying with U.S. Environmental Protection Agency 2007 Emissions Standards. J. Air Waste Manag. Assoc. 2011, 61, 427–442.
- Khalek, I.A.; Blanks, M.G.; Merritt, P.M.; Zielinska, B. Regulated and unregulated emissions from modern 2010 emissions-compliant heavy-duty on-highway diesel engines. J. Air Waste Manag. Assoc. 2015, 65, 987–1001.
- Zeraati-Rezaei, S.; Alam, M.S.; Xu, H.; Beddows, D.C.; Harrison, R.M. Size-resolved physico-chemical characterization of diesel exhaust particles and efficiency of exhaust aftertreatment. Atmos. Environ. 2019, 222, 117021.
- Huang, L.; Bohac, S.V.; Chernyak, S.M.; Batterman, S.A. Effects of fuels, engine load and exhaust after-treatment on diesel engine SVOC emissions and development of SVOC profiles for receptor modeling. Atmos. Environ. 2014, 102, 228–238.
- Alanen, J.; Simonen, P.; Saarikoski, S.; Timonen, H.; Kangasniemi, O.; Saukko, E.; Hillamo, R.; Lehtoranta, K.; Murtonen, T.; Vesala, H.; et al. Comparison of primary and secondary particle formation from natural gas engine exhaust and of their volatility characteristics. Atmos. Chem. Phys. 2017, 17, 8739–8755.
- Li, Y.; Xue, J.; Peppers, J.; Kado, N.Y.; Vogel, C.F.; Alaimo, C.P.; Green, P.G.; Zhang, R.; Jenkins, B.M.; Kim, M.; et al. Chemical and Toxicological Properties of Emissions from a Light-Duty Compressed Natural Gas Vehicle Fueled with Renewable Natural Gas. Environ. Sci. Technol. 2021, 55, 2820–2830.
- Rönkkö, T.; Virtanen, A.; Lehtoranta, K.; Keskinen, J.; Pirjola, L.; Lappi, M. Effect of dilution conditions and driving parameters on nucleation mode particles in diesel exhaust: Laboratory and on-road study. Atmos. Environ. 2006, 40, 2893–2901.
- Ntziachristos, L.; Ning, Z.; Geller, M.D.; Sioutas, C. Particle Concentration and Characteristics near a Major Freeway with Heavy-Duty Diesel Traffic. Environ. Sci. Technol. 2007, 41, 2223–2230.
- Zhang, K.M.; Wexler, A.S. Evolution of particle number distribution near roadways—Part I: Analysis of aerosol dynamics and its implications for engine emission measurement. Atmos. Environ. 2004, 38, 6643–6653.
- Kwak, J.H.; Kim, H.S.; Lee, J.H.; Lee, S.H. On-road chasing measurement of exhaust particle emissions from diesel, CNG, LPG, and DME-fueled vehicles using a mobile emission laboratory. Int. J. Automot. Technol. 2014, 15, 543–551.
- Shen, X.; Yao, Z.; He, K.; Cao, X.; Liu, H. The Construction and Application of a Multipoint Sampling System for Vehicle Exhaust Plumes. Aerosol Air Qual. Res. 2017, 17, 1705–1716.
- Sasaki, S.; Nakajima, T. Study on the Measuring Method of Vehicular PM Size Distribution to Simulate the Atmospheric Dilution Process; SAE International: Warrendale, PA, USA, 2002.
- Lee, S.H.; Kwak, J.H.; Lee, J.H. On-road chasing and laboratory measurements of exhaust particle emissions of diesel vehicles equipped with aftertreatment technologies (DPF, urea-SCR). Int. J. Automot. Technol. 2015, 16, 551–559.
- Harrison, R.M.; MacKenzie, A.R.; Xu, H.; Alam, M.S.; Nikolova, I.; Zhong, J.; Singh, A.; Zeraati-Rezaei, S.; Stark, C.; Beddows, D.; et al. Diesel exhaust nanoparticles and their behaviour in the atmosphere. Proc. R. Soc. A Math. Phys. Eng. Sci. 2018, 474, 20180492.
- Saha, P.K.; Khlystov, A.; Snyder, M.G.; Grieshop, A.P. Characterization of air pollutant concentrations, fleet emission factors, and dispersion near a North Carolina interstate freeway across two seasons. Atmos. Environ. 2018, 177, 143–153.
- Choi, W.; Paulson, S.E. Closing the ultrafine particle number concentration budget at road-to-ambient scale: Implications for particle dynamics. Aerosol Sci. Technol. 2016, 50, 448–461.
- Fushimi, A.; Hasegawa, S.; Takahashi, K.; Fujitani, Y.; Tanabe, K.; Kobayashi, S. Atmospheric fate of nuclei-mode particles estimated from the number concentrations and chemical composition of particles measured at roadside and background sites. Atmos. Environ. 2008, 42, 949–959.
- Zimmerman, N.; Wang, J.M.; Jeong, C.-H.; Ramos, M.; Hilker, N.; Healy, R.M.; Sabaliauskas, K.; Wallace, J.S.; Evans, G.J. Field Measurements of Gasoline Direct Injection Emission Factors: Spatial and Seasonal Variability. Environ. Sci. Technol. 2016, 50, 2035–2043.
- Kangasniemi, O.; Kuuluvainen, H.; Heikkilä, J.; Pirjola, L.; Niemi, J.V.; Timonen, H.; Saarikoski, S.; Rönkkö, T.; Maso, M.D. Dispersion of a Traffic Related Nanocluster Aerosol Near a Major Road. Atmosphere 2019, 10, 309.
- Kumar, P.; Ketzel, M.; Vardoulakis, S.; Pirjola, L.; Britter, R. Dynamics and dispersion modelling of nanoparticles from road traffic in the urban atmospheric environment—A review. J. Aerosol Sci. 2011, 42, 580–603.
- Carpentieri, M.; Kumar, P. Ground-fixed and on-board measurements of nanoparticles in the wake of a moving vehicle. Atmos. Environ. 2011, 45, 5837–5852.
- Liu, H.; Qi, L.; Liang, C.; Deng, F.; Man, H.; He, K. How aging process changes characteristics of vehicle emissions? A review. Crit. Rev. Environ. Sci. Technol. 2019, 50, 1796–1828.
- Hidy, G.M. Atmospheric Chemistry in a Box or a Bag. Atmosphere 2019, 10, 401.
- Ahlberg, E.; Ausmeel, S.; Eriksson, A.; Holst, T.; Karlsson, T.; Brune, W.H.; Frank, G.; Roldin, P.; Kristensson, A.; Svenningsson, B. No Particle Mass Enhancement from Induced Atmospheric Ageing at a Rural Site in Northern Europe. Atmosphere 2019, 10, 408.
- Suarez-Bertoa, R.; Zardini, A.; Platt, S.; Hellebust, S.; Pieber, S.M.; El Haddad, I.; Temime-Roussel, B.; Baltensperger, U.; Marchand, N.; Prevot, A.; et al. Primary emissions and secondary organic aerosol formation from the exhaust of a flex-fuel (ethanol) vehicle. Atmos. Environ. 2015, 117, 200–211.
- Karjalainen, P.; Timonen, H.; Saukko, E.; Kuuluvainen, H.; Saarikoski, S.; Aakko-Saksa, P.; Murtonen, T.; Bloss, M.; Maso, M.D.; Simonen, P.; et al. Time-resolved characterization of primary particle emissions and secondary particle formation from a modern gasoline passenger car. Atmos. Chem. Phys. 2016, 16, 8559–8570.
- Roth, P.; Yang, J.; Peng, W.; Cocker, D.R.; Durbin, T.D.; Asa-Awuku, A.; Karavalakis, G. Intermediate and high ethanol blends reduce secondary organic aerosol formation from gasoline direct injection vehicles. Atmos. Environ. 2019, 220, 117064.
- Chirico, R.; Clairotte, M.; Adam, T.W.; Giechaskiel, B.; Heringa, M.F.; Elsasser, M.; Martini, G.; Manfredi, U.; Streibel, T.; Sklorz, M.; et al. Emissions of Organic Aerosol Mass, Black Carbon, Particle Number, and Regulated and Unregulated Gases from Scooters and Light and Heavy Duty Vehicles with Different Fuels. Atmos. Chem. Phys. Discuss. 2014, 14, 16591–16639.
- Deng, W.; Hu, Q.; Liu, T.; Wang, X.; Zhang, Y.; Song, W.; Sun, Y.; Bi, X.; Yu, J.; Yang, W.; et al. Primary particulate emissions and secondary organic aerosol (SOA) formation from idling diesel vehicle exhaust in China. Sci. Total Environ. 2017, 593–594, 462–469.
- Zhao, Y.; Lambe, A.T.; Saleh, R.; Saliba, G.; Robinson, A.L. Secondary Organic Aerosol Production from Gasoline Vehicle Exhaust: Effects of Engine Technology, Cold Start, and Emission Certification Standard. Environ. Sci. Technol. 2018, 52, 1253–1261.
- Vu, D.; Roth, P.; Berte, T.; Yang, J.; Cocker, D.; Durbin, T.D.; Karavalakis, G.; Asa-Awuku, A. Using a new Mobile Atmospheric Chamber (MACh) to investigate the formation of secondary aerosols from mobile sources: The case of gasoline direct injection vehicles. J. Aerosol Sci. 2019, 133, 1–11.
- Du, Z.; Hu, M.; Peng, J.; Zhang, W.; Zheng, J.; Gu, F.; Qin, Y.; Yang, Y.; Li, M.; Wu, Y.; et al. Comparison of primary aerosol emission and secondary aerosol formation from gasoline direct injection and port fuel injection vehicles. Atmos. Chem. Phys. 2018, 18, 9011–9023.
- Gordon, T.D.; Presto, A.A.; May, A.A.; Nguyen, N.T.; Lipsky, E.M.; Donahue, N.M.; Gutierrez, A.; Zhang, M.; Maddox, C.; Rieger, P.; et al. Secondary organic aerosol formation exceeds primary particulate matter emissions for light-duty gasoline vehicles. Atmos. Chem. Phys. 2014, 14, 4661–4678.
- Gentner, D.R.; Jathar, S.H.; Gordon, T.D.; Bahreini, R.; Day, D.; El Haddad, I.; Hayes, P.L.; Pieber, S.M.; Platt, S.; de Gouw, J.; et al. Review of Urban Secondary Organic Aerosol Formation from Gasoline and Diesel Motor Vehicle Emissions. Environ. Sci. Technol. 2017, 51, 1074–1093.
- Timonen, H.; Karjalainen, P.; Saukko, E.; Saarikoski, S.; Aakko-Saksa, P.; Simonen, P.; Murtonen, T.; Maso, M.D.; Kuuluvainen, H.; Bloss, M.; et al. Influence of fuel ethanol content on primary emissions and secondary aerosol formation potential for a modern flex-fuel gasoline vehicle. Atmos. Chem. Phys. 2017, 17, 5311–5329.
- Karjalainen, P.; Rönkkö, T.; Simonen, P.; Ntziachristos, L.; Juuti, P.; Timonen, H.; Teinilä, K.; Saarikoski, S.; Saveljeff, H.; Lauren, M.; et al. Strategies to Diminish the Emissions of Particles and Secondary Aerosol Formation from Diesel Engines. Environ. Sci. Technol. 2019, 53, 10408–10416.
- Gramsch, E.; Papapostolou, V.; Reyes, F.; Vásquez, Y.; Castillo, M.; Oyola, P.; López, G.; Cádiz, A.; Ferguson, S.; Wolfson, M.; et al. Variability in the primary emissions and secondary gas and particle formation from vehicles using bioethanol mixtures. J. Air Waste Manag. Assoc. 2018, 68, 329–346.
- May, A.A.; Nguyen, N.T.; Presto, A.A.; Gordon, T.; Lipsky, E.M.; Karve, M.; Gutierrez, A.; Robertson, W.H.; Zhang, M.; Brandow, C.; et al. Gas- and particle-phase primary emissions from in-use, on-road gasoline and diesel vehicles. Atmos. Environ. 2014, 88, 247–260.
- Gordon, T.D.; Presto, A.A.; Nguyen, N.T.; Robertson, W.H.; Na, K.; Sahay, K.N.; Zhang, M.; Maddox, C.; Rieger, P.; Chattopadhyay, S.; et al. Secondary organic aerosol production from diesel vehicle exhaust: Impact of aftertreatment, fuel chemistry and driving cycle. Atmos. Chem. Phys. 2014, 14, 4643–4659.
- Mehsein, K.; Norsic, C.; Chaillou, C.; Nicolle, A. Minimizing secondary pollutant formation through identification of most influential volatile emissions in gasoline exhausts: Impact of the vehicle powertrain technology. Atmos. Environ. 2020, 226, 117394.
- Park, G.; Kim, K.; Park, T.; Kang, S.; Ban, J.; Choi, S.; Yu, D.-G.; Lee, S.; Lim, Y.; Kim, S.; et al. Primary and secondary aerosols in small passenger vehicle emissions: Evaluation of engine technology, driving conditions, and regulatory standards. Environ. Pollut. 2021, 286, 117195.
- Jathar, S.H.; Friedman, B.; Galang, A.A.; Link, M.F.; Brophy, P.; Volckens, J.; Eluri, S.; Farmer, D.K. Linking Load, Fuel, and Emission Controls to Photochemical Production of Secondary Organic Aerosol from a Diesel Engine. Environ. Sci. Technol. 2017, 51, 1377–1386.
- Bahreini, R.; Middlebrook, A.; de Gouw, J.; Warneke, C.; Trainer, M.; Brock, C.A.; Stark, H.; Brown, S.S.; Dube, W.P.; Gilman, J.B.; et al. Gasoline emissions dominate over diesel in formation of secondary organic aerosol mass. Geophys. Res. Lett. 2012, 39, L06805.
- Platt, S.M.; El Haddad, I.; Zardini, A.A.; Clairotte, M.; Astorga, C.; Wolf, R.; Slowik, J.G.; Temime-Roussel, B.; Marchand, N.; Ježek, I.; et al. Secondary organic aerosol formation from gasoline vehicle emissions in a new mobile environmental reaction chamber. Atmos. Chem. Phys. 2013, 13, 9141–9158.
- Pieber, S.M.; Kumar, N.K.; Klein, F.; Comte, P.; Bhattu, D.; Dommen, J.; Bruns, E.A.; Kılıç, D.; El Haddad, I.; Keller, A.; et al. Gas-phase composition and secondary organic aerosol formation from standard and particle filter-retrofitted gasoline direct injection vehicles investigated in a batch and flow reactor. Atmos. Chem. Phys. 2018, 18, 9929–9954.
- Kuittinen, N.; McCaffery, C.; Peng, W.; Zimmerman, S.; Roth, P.; Simonen, P.; Karjalainen, P.; Keskinen, J.; Cocker, D.R.; Durbin, T.D.; et al. Effects of driving conditions on secondary aerosol formation from a GDI vehicle using an oxidation flow reactor. Environ. Pollut. 2021, 282, 117069.
- Kuittinen, N.; McCaffery, C.; Zimmerman, S.; Bahreini, R.; Simonen, P.; Karjalainen, P.; Keskinen, J.; Rönkkö, T.; Karavalakis, G. Using an oxidation flow reactor to understand the effects of gasoline aromatics and ethanol levels on secondary aerosol formation. Environ. Res. 2021, 200, 111453.
- Giechaskiel, B.; Joshi, A.; Ntziachristos, L.; Dilara, P. European Regulatory Framework and Particulate Matter Emissions of Gasoline Light-Duty Vehicles: A Review. Catalysts 2019, 9, 586.
- Kontses, A.; Ntziachristos, L.; Zardini, A.; Papadopoulos, G.; Giechaskiel, B. Particulate emissions from L-Category vehicles towards Euro 5. Environ. Res. 2019, 182, 109071.
- Giechaskiel, B.; Zardini, A.A.; Lähde, T.; Perujo, A.; Kontses, A.; Ntziachristos, L. Particulate Emissions of Euro 4 Motorcycles and Sampling Considerations. Atmosphere 2019, 10, 421.
- Bagley, S.T.; Baumgard, K.; Gratz, L.; Johnson, J.H.; Leddy, D. Haracterization of Fuel and Aftertreatment Device Effects on Diesel Emissions. Res. Rep. Health Eff. Inst. 1996, 76, 1–75.
- Vouitsis, E.; Ntziachristos, L.; Samaras, Z. Theoretical Investigation of the Nucleation Mode Formation Downstream of Diesel After-treatment Devices. Aerosol Air Qual. Res. 2008, 8, 37–53.
- de Jesus, A.L.; Rahman, M.; Mazaheri, M.; Thompson, M.; Knibbs, L.; Jeong, C.; Evans, G.; Nei, W.; Ding, A.; Qiao, L.; et al. Ultrafine particles and PM2.5 in the air of cities around the world: Are they representative of each other? Environ. Int. 2019, 129, 118–135.
- Mohr, M.; Lehmann, U.; Margaria, G. ACEA Programme on the Emissions of Fine Particulates from Passenger Cars(2) Part1: Particle Characterisation of a Wide Range of Engine Technologies; SAE International: Warrendale, PA, USA, 2003.
- Ntziachristos, L.; Mamakos, A.; Samaras, Z.; Mathis, U.; Mohr, M.; Thompson, N.; Stradling, R.; Forti, L.; De Serves, C. Overview of the European “Particulates” Project on the Characterization of Exhaust Particulate Emissions from Road Vehicles: Results for Light-Duty Vehicles; SAE International: Warrendale, PA, USA, 2004.
- Giechaskiel, B.; Vanhanen, J.; Väkevä, M.; Martini, G. Investigation of vehicle exhaust sub-23 nm particle emissions. Aerosol Sci. Technol. 2017, 51, 626–641.
- Xue, J.; Li, Y.; Quiros, D.; Hu, S.; Huai, T.; Ayala, A.; Jung, H.S. Investigation of alternative metrics to quantify PM mass emissions from light duty vehicles. J. Aerosol Sci. 2017, 113, 85–94.
- Kontses, A.; Triantafyllopoulos, G.; Ntziachristos, L.; Samaras, Z. Particle number (PN) emissions from gasoline, diesel, LPG, CNG and hybrid-electric light-duty vehicles under real-world driving conditions. Atmos. Environ. 2019, 222, 117126.
- Li, W.; Collins, J.F.; Norbeck, J.M.; Cocker, D.R.; Sawant, A. Assessment of Particulate Matter Emissions from a Smple of In-Use ULEV and SULEV Vehicles; SAE International: Warrendale, PA, USA, 2006.
- Badshah, H.; Kittelson, D.; Northrop, W. Particle Emissions from Light-Duty Vehicles during Cold-Cold Start. SAE Int. J. Engines 2016, 9, 1775–1785.
- Karavalakis, G.; Short, D.; Chen, V.; Espinoza, C.; Berte, T.; Durbin, T.; Asa-Awuku, A.; Jung, H.; Ntziachristos, L.; Amanatidis, S.; et al. Evaluating Particulate Emissions from a Flexible Fuel Vehicle with Direct Injection when Operated on Ethanol and Iso-Butanol Blends; SAE International: Warrendale, PA, USA, 2014.
- Maricq, M.M.; Szente, J.J.; Harwell, A.L.; Loos, M.J. Impact of aggressive drive cycles on motor vehicle exhaust PM emissions. J. Aerosol Sci. 2017, 113, 1–11.
- Hu, Z.; Lu, Z.; Song, B.; Quan, Y. Impact of test cycle on mass, number and particle size distribution of particulates emitted from gasoline direct injection vehicles. Sci. Total Environ. 2020, 762, 143128.
- Larsson, T.; Prasath, A.; Olofsson, U.; Erlandsson, A. Undiluted Measurement of Sub 10 nm Non-Volatile and Volatile Particle Emissions from a DISI Engine Fueled with Gasoline and Ethanol; SAE International: Warrendale, PA, USA, 2021.
- Di Iorio, S.; Catapano, F.; Magno, A.; Sementa, P.; Vaglieco, B.M. Investigation on sub-23 nm particles and their volatile organic fraction (VOF) in PFI/DI spark ignition engine fueled with gasoline, ethanol and a 30% v/v ethanol blend. J. Aerosol Sci. 2020, 153, 105723.
- Samaras, Z.C.; Andersson, J.; Bergmann, A.; Hausberger, S.; Toumasatos, Z.; Keskinen, J.; Haisch, C.; Kontses, A.; Ntziachristos, L.D.; Landl, L.; et al. Measuring Automotive Exhaust Particles Down to 10 nm. SAE Int. J. Adv. Curr. Pract. Mobil. 2020, 3, 539–550.
- Li, C.; Swanson, J.; Pham, L.; Hu, S.; Hu, S.; Mikailian, G.; Jung, H.S. Real-world particle and NOx emissions from hybrid electric vehicles under cold weather conditions. Environ. Pollut. 2021, 286, 117320.
- Thompson, N.; Ntziachristos, L.; Samaras, Z.; Aakko, P.; Wass, U.; Hausberger, S.; Sams, T. Overview of the European “Particulates” Project on the Characterization of Exhaust Particulate Emissions from Road Vehicles: Results for Heavy Duty Engines; SAE International: Warrendale, PA, USA, 2004.
- Wang, T.; Quiros, D.C.; Thiruvengadam, A.; Pradhan, S.; Hu, S.; Huai, T.; Lee, E.S.; Zhu, Y. Total Particle Number Emissions from Modern Diesel, Natural Gas, and Hybrid Heavy-Duty Vehicles During On-Road Operation. Environ. Sci. Technol. 2017, 51, 6990–6998.
- Swanson, J.J.; Kittelson, D.B.; Watts, W.F.; Gladis, D.D.; Twigg, M.V. Influence of storage and release on particle emissions from new and used CRTs. Atmos. Environ. 2009, 43, 3998–4004.
- Giechaskiel, B.; Zardini, A.; Martini, G. Particle Emission Measurements from L-Category Vehicles. SAE Int. J. Engines 2015, 8, 2322–2337.
- Premnath, V.; Zavala, B.; Khalek, I.; Eakle, S.; Henry, C. Detailed Characterization of Particle Emissions from Advanced Internal Combustion Engines; SAE International: Warrendale, PA, USA, 2021.
- Yamada, H.; Inomata, S.; Tanimoto, H. Mechanisms of Increased Particle and VOC Emissions during DPF Active Regeneration and Practical Emissions Considering Regeneration. Environ. Sci. Technol. 2017, 51, 2914–2923.
- Giechaskiel, B. Particle Number Emissions of a Diesel Vehicle during and between Regeneration Events. Catalysts 2020, 10, 587.
- R’Mili, B.; Boréave, A.; Meme, A.; Vernoux, P.; Leblanc, M.; Noël, L.; Raux, S.; D’Anna, B. Physico-Chemical Characterization of Fine and Ultrafine Particles Emitted during Diesel Particulate Filter Active Regeneration of Euro5 Diesel Vehicles. Environ. Sci. Technol. 2018, 52, 3312–3319.
- Leblanc, M.; Noel, L.; R’Mili, B.; Boréave, A.; D’Anna, B.; Raux, S. Impact of Engine Warm-up and DPF Active Regeneration on Regulated & Unregulated Emissions of a Euro 6 Diesel SCR Equipped Vehicle. J. Earth Sci. Geotech. Eng. 2016, 6, 29–50.
- Transport & Environment. New Diesels, New Problems; European Federation for Transport and Environment AISBL: Brussels, Belgium, 2020.
- Giechaskiel, B.; Lahde, T.; Suarez-Bertoa, R.; Clairotte, M.; Grigoratos, T.; Zardini, A.; Perujo, A.; Martini, G. Particle number measurements in the European legislation and future JRC activities. Combust. Engines 2018, 174, 3–16.
- Giechaskiel, B.; Gioria, R.; Carriero, M.; Lähde, T.; Forloni, F.; Perujo, A.; Martini, G.; Bissi, L.M.; Terenghi, R. Emission Factors of a Euro VI Heavy-duty Diesel Refuse Collection Vehicle. Sustainability 2019, 11, 1067.
- Valverde, V.; Giechaskiel, B. Assessment of Gaseous and Particulate Emissions of a Euro 6d-Temp Diesel Vehicle Driven >1300 km Including Six Diesel Particulate Filter Regenerations. Atmosphere 2020, 11, 645.
- Bikas, G.; Zervas, E. Regulated and Non-Regulated Pollutants Emitted during the Regeneration of a Diesel Particulate Filter. Energy Fuels 2007, 21, 1543–1547.
