It is an understatement that mating and DNA transfer are key events for living organisms. Among the traits needed to facilitate mating, cell adhesion between gametes is a universal requirement. Thus, there should be specific properties for the adhesion proteins involved in mating. Biochemical and biophysical studies have revealed structural information about mating adhesins, as well as their specificities and affinities, leading to some ideas about these specialized adhesion proteins. Single-cell force spectroscopy (SCFS) has added important findings. In SCFS, mating cells are brought into contact in an atomic force microscope (AFM), and the adhesive forces are monitored through the course of mating. The results have shown some remarkable characteristics of mating adhesins and add knowledge about the design and evolution of mating adhesins.
- atomic force microscopy
- cell–cell mating
- adhesion
- single-cell force spectroscopy
- yeasts
- bacteria
- conjugation
- gametes
1. Introduction
2. Characteristics of SCFS
Single-cell force spectroscopy (SCFS) has been instrumental in improving our understanding of cell–substrate adhesion, cell–cell adhesion, and cell–host adhesion down to the molecular level (for recent reviews, herwein direct the reader toward [6][8][6,8]). Briefly, the principle of SCFS consists of attaching a single cell to a soft, force-sensitive AFM cantilever (Figure 12a). This cell probe can then be used to measure interactions with other surfaces, including those of other single cells. By bringing the cell probe in contact with the surface of another cell and subsequently retracting it away, while recording the forces between the two cells, force–distance (FD) curves are generated, from which the strength of adhesion between the two cells can be determined accurately. Importantly, the velocity at which the cell probe is approached and pulled away from the sampled cell can also be controlled precisely. For most measurements, an arbitrary constant approach/retract velocity of ~1–10 µm·s−1 is used to roughly match physiological conditions [9][37]. However, varying the retraction velocity allows assessing the effect of different force loading rates on the tensile strength of molecular complexes formed between adhesive molecules on the surfaces of the interacting cells. Performing such dynamic force spectroscopy experiments can provide information on the energy landscape underlying forced unbinding of receptor–ligand complexes [10][38]. AFM can also be utilized to investigate the binding dynamics between single cells and polypeptide ligands. ThWe authors previously used this technique (called single-molecule force spectroscopy, AFM-SMFS) to detect and analyze the unfolding of C. albicans Als5p. In those experiments, a single yeast cell expressing a V5 epitope-tagged Als5p was immobilized onto a polymer membrane. Als5p-V5 was detected, and the adhesion forces were measured using AFM tips coupled to anti-V5 antibodies [11][39]. Notably, recent AFM-SCFS experiments have revealed binding dynamics associated between the SARS-CoV-2 spike protein and human ACE2 receptor. In those experiments, the receptor-binding domain (RBD) of the SARS-CoV-2 spike protein was attached to the AFM tip, and the purified ACE2 receptor or ACE2-expressing cells were immobilized onto their respective surface [12][13][40,41].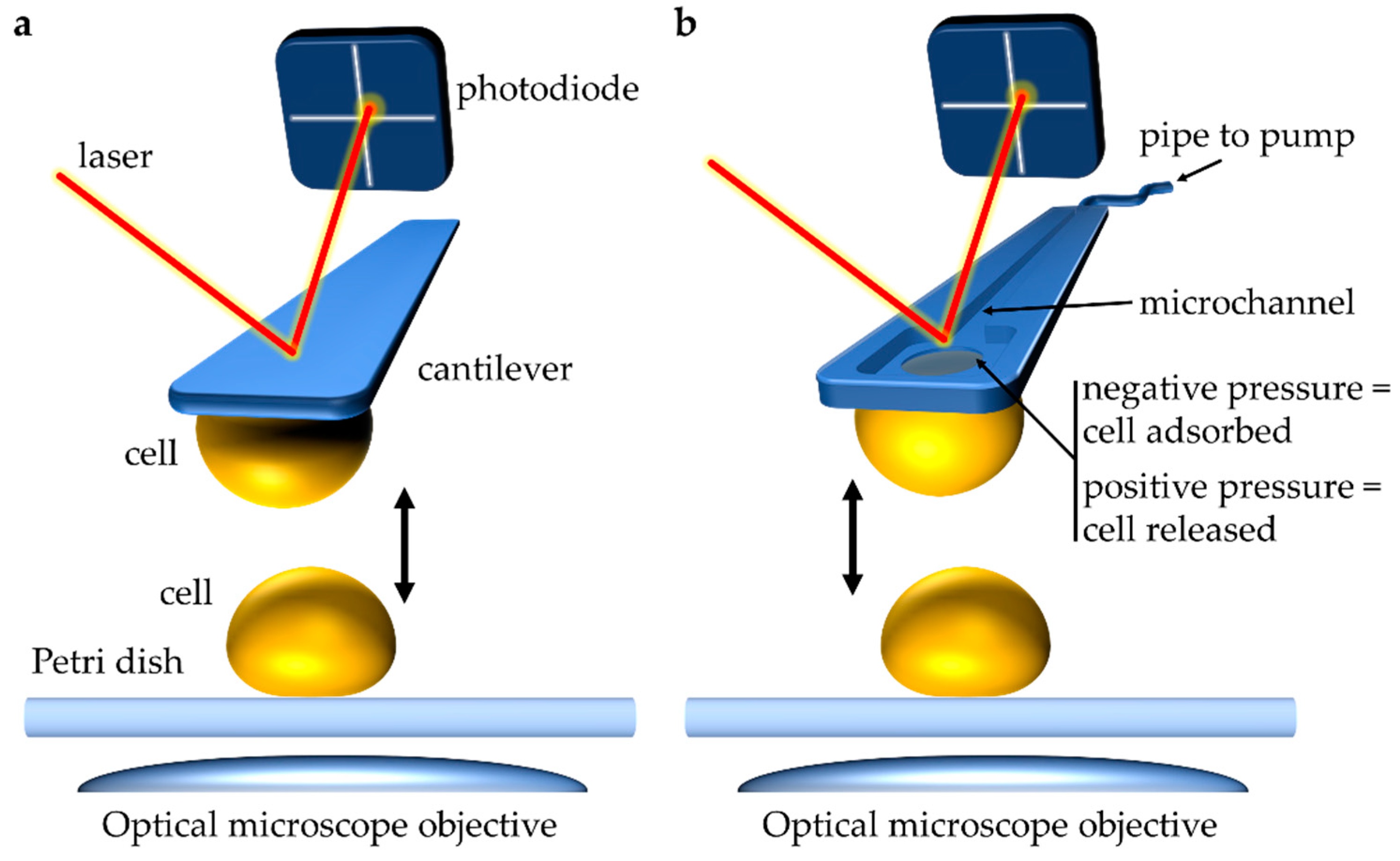
2.1. SCFS Application to Studying Yeasts
2.1.1. Intercellular Adhesion
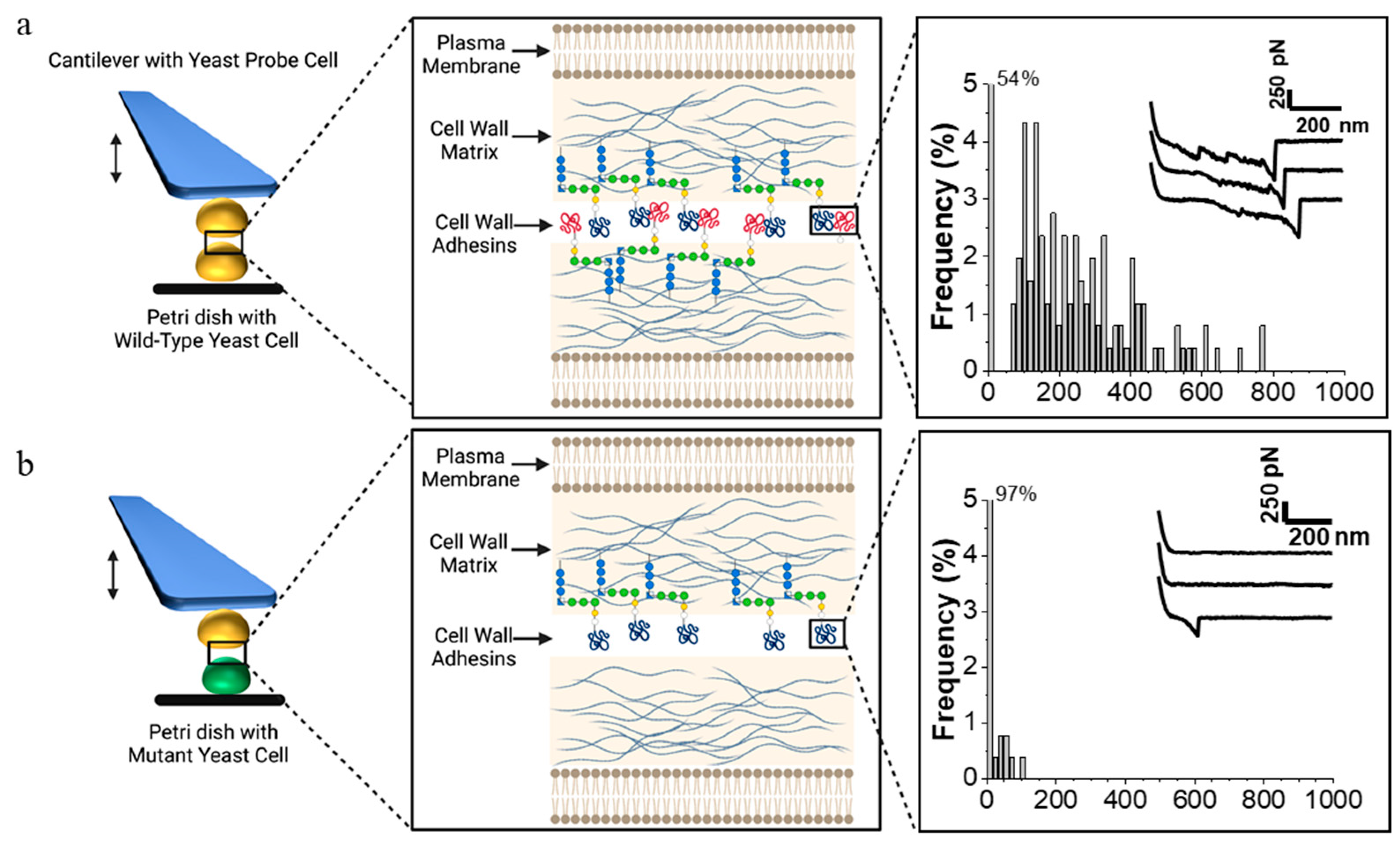
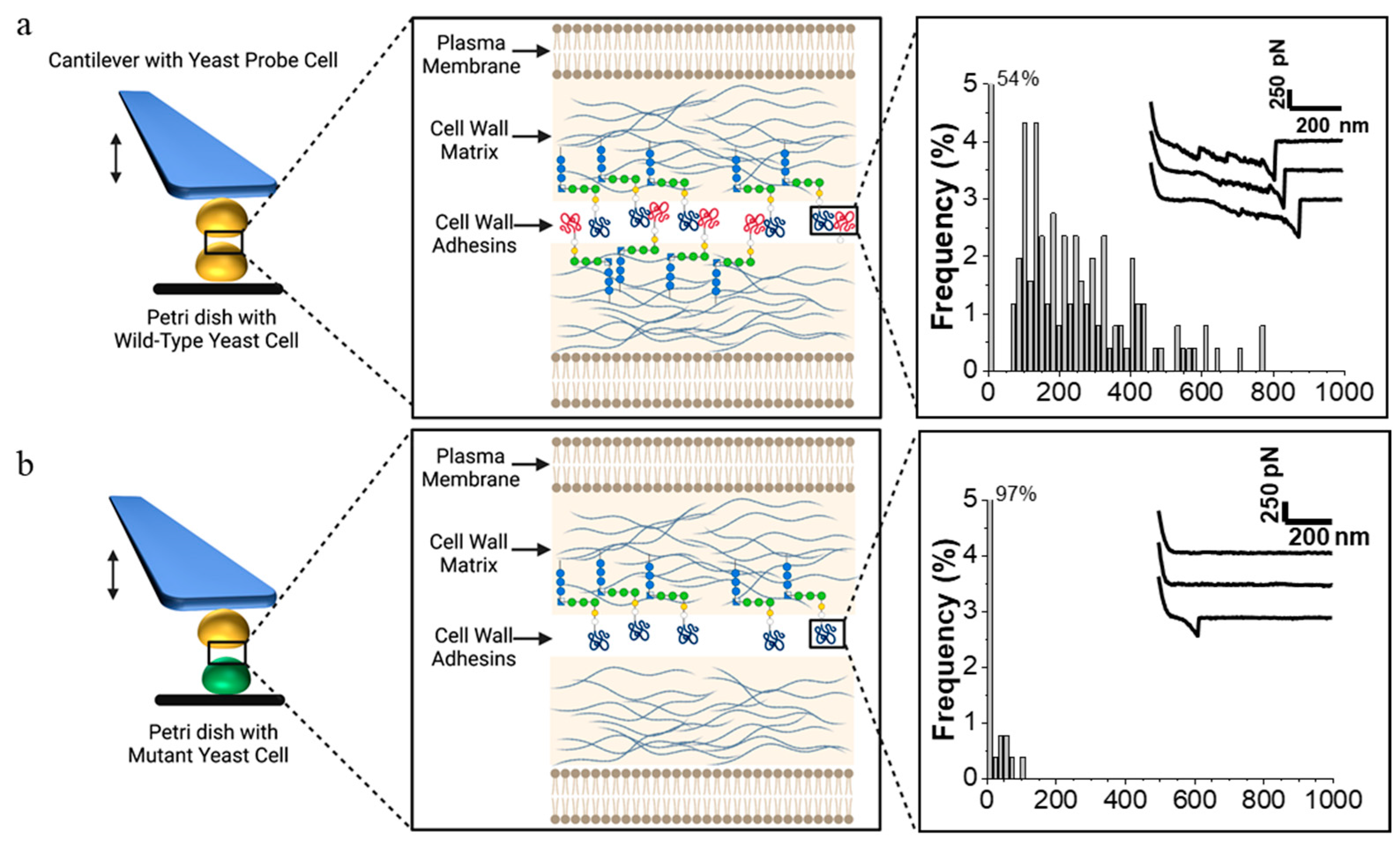
2.1.2. Intercellular Adhesion during Mating
2.2. SCFS Application to Studying Bacteria
2.2.1. Bacterial Adhesion
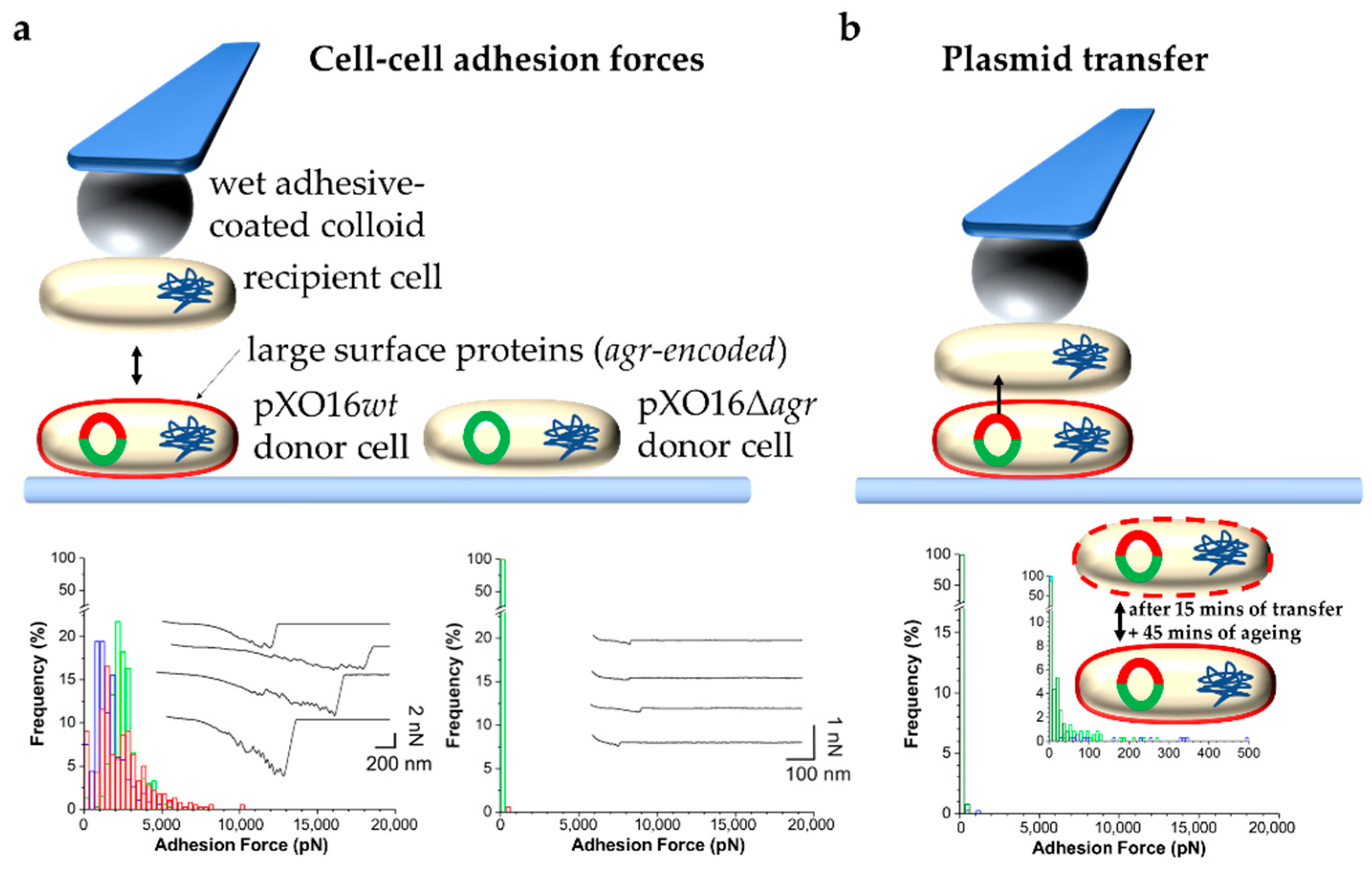
2.2.2. Bacterial Adhesion in the Context of Mating