You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Franco Bandi and Version 1 by Franco Bandi.
Sensors based on single-photon avalanche diodes (SPADs) are nowadays employed in a wide variety of single-photon counting and fast-timing applications, e.g., high-energy physics; time of flight (TOF) ranging and 3D imaging; Raman spectroscopy; and bio-medicine, including fluorescence-lifetime imaging microscopy and positron emission tomography (PET), to name a few. SiPMs are the most common sensors in PET applications, where they detect the light produced by the interaction between gamma photons and scintillator crystals.
- silicon photomultipliers
1. Introduction
Sensors based on single-photon avalanche diodes (SPADs) are nowadays employed in a wide variety of single-photon counting and fast-timing applications, e.g., high-energy physics [1,2]; time of flight (TOF) ranging and 3D imaging [3]; Raman spectroscopy [4]; and bio-medicine, including fluorescence-lifetime imaging microscopy [5,6] and positron emission tomography (PET) [7,8,9], to name a few. When implemented in CMOS image sensor technologies, SPAD sensor architectures benefit from the combination of per-pixel and per-chip processing and control circuitry with good-enough photodetectors [7,10,11]. Particularly, as Figure 1 illustrates, digital SiPMs employ micro-cells consisting of SPADs and embedded processing circuitry to directly encode SPAD avalanches into digital values, thus providing large flexibility for system implementation [12].
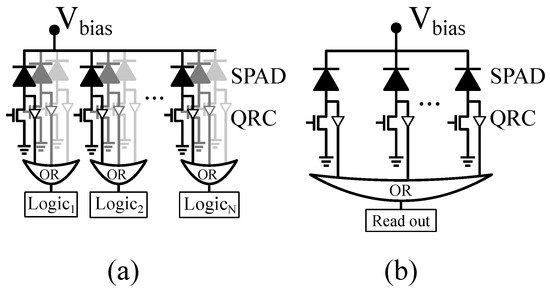
Figure 1. (a) Segmented digital silicon photomultiplier (SiPM) and (b) digital SiPM with common readout.
SiPMs are the most common sensors in PET applications, where they detect the light produced by the interaction between gamma photons and scintillator crystals, as it is schematically shown in Figure 2. Even though for this analysis, we will focus on monolithic crystal blocks attached to an array of SiPMs [13], the issues encountered for this configuration can be partially extrapolated to detectors consisting of pixelated crystals [14,15,16,17]. For a monolithic crystal, the scintillation photons are spread all over the array and, as a result, the amount of photons over each sensor is relatively small. These optical photons contain information about the arrival time of the incident particle, the energy transfer to the crystal, and the first interaction point within the crystal. Therefore, it is essential to maximize their detection to improve the reconstruction of the gamma event and, ultimately, the quality of the PET image.
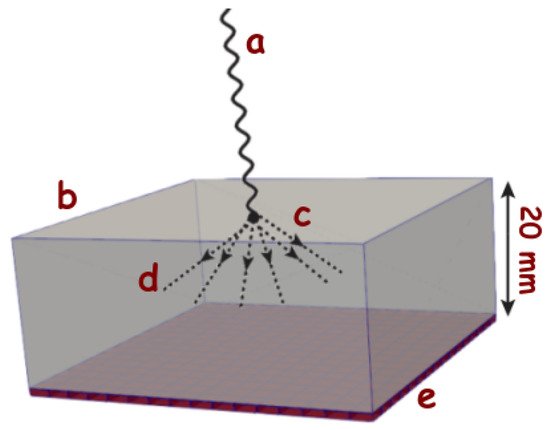
Figure 2. Schematical representation of an incident gamma ray (a) that interacts with a scintillating crystal (b) of typical size (50 × 50 × 20 mm2) at the point (c), producing optical photons (d) (which are of the order of 105/MeV depending on the crystal) that are detected by the photodetector placed at (e) that is optically coupled (i.e., with optical grease) to the scintillator.
For pixelated crystal detector configurations, the intrinsic spatial resolution is determined by the pixel’s size. However, as the gamma photon normally suffers from Compton scattering before being totally absorbed, the optical photons are spread over more than one SiPM pixel, similar to the previous case. Hence, for this configuration also, we need a good timing resolution in order to determine the first interaction point and reduce the Compton noise that affects the resolution of the reconstructed medical image. In addition, both pixelated and monolithic detectors require good timing resolution to achieve satisfactory coincidence time resolution (CTR) and enable the inclusion of TOF information for PET scanners. This can be achieved with a smaller SiPM pixel size with independent TDCs.
2. Trade-Offs in SiPM Design for PET and SPECT
For PET and SPECT imaging, one needs to reconstruct the interaction point in the scintillating crystal. However, before a gamma ray is totally absorbed through a photoelectric effect, it usually undergoes previous Compton scattering in a non-negligible percentage of cases. In Figure 3, we see (from a Monte Carlo simulation) a typical example of Compton scattering (C) previous to a photoelectric absorption (P). Ideally, in order to perform an accurate image reconstruction, one would need to extract the first interaction point (C). However, with current technology, this is a rather challenging task, and the estimated interaction point normally corresponds to the center of mass (CM) of the energy distribution of the optical photon readout. A good timing resolution together with an independent timestamp readout of each pixel would allow us to disentangle the two interactions and their chronological order and therefore recognize the first interaction point. We shall briefly comment on these aspects in the following paragraphs.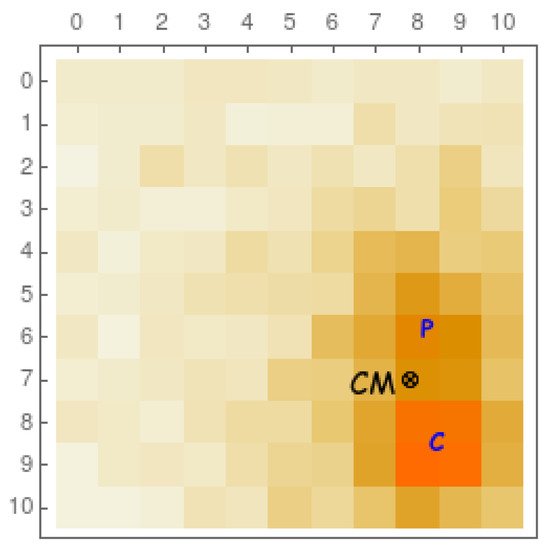
Figure 3. Representation of a 10×10 10 x 10 matrix of 1 × 1 mm2 pixels (coupled to a scintillating crystal as in Figure 2, but with a smaller size) for a typical event with a Compton interaction (C) previous to a photoelectric absorption (P). The electronic readout normally associates the coordinates of this gamma ray event to the center of mass of the energy distribution (CM). Higher-intensity colors correspond to a higher number of detected optical photons.
