Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Catherine Yang and Version 1 by Xinxin Zhang.
Iron is an essential element for plant growth and development. While abundant in soil, the available Fe in soil is limited. In this regard, plants have evolved a series of mechanisms for efficient iron uptake, allowing plants to better adapt to iron deficient conditions. These mechanisms include iron acquisition from soil, iron transport from roots to shoots, and iron storage in cells. The mobilization of Fe in plants often occurs via chelating with phytosiderophores, citrate, nicotianamine, mugineic acid, or in the form of free iron ions.
- iron deficiency
1. Introduction
Iron (Fe) is an essential micronutrient for plant growth development and plays a key role in regulating numerous cellular processes. Iron, as an important co-factor for enzymes, plays an important role in regulating plant photosynthesis, mitochondrial respiration, the synthesis and repair of nucleotides, and metal homeostasis, especially in the maintenance of structural integrity of various proteins [1]. While Fe is abundant in soil, the available Fe in soil for plants is often insufficient, particularly in calcareous soils, due to low solubility of Fe. Iron deficiency is one of the most important factors limiting crop production in the world. Plants grown in low Fe soils often exhibit chlorosis and decreased photosynthesis, leading to reduction in yield and quality of crops. To cope with this situation, plants have evolved a series of sophisticate mechanisms to adapt to iron-deficient conditions in soil. In addition, iron deficiency is a significant worldwide problem, seriously affecting over 30% of the world’s population (http://www.who.int/nutrition/topics/ida/en/). Anemia as one of the severest nutritional disorders is caused by low iron in humans. Therefore, elucidation of the molecular and physiological mechanisms by which plants sense, respond, and adapt to Fe deficiency would contribute to cultivating crop varieties with high Fe efficiency.
2. Iron Acquisition from Soil to Roots
Although iron is considered as the fourth most abundant element, one-third of soil on the Earth is estimated as Fe deficient [1]. The solubility and availability of iron in soil can be affected by multiple factors, including soil pH, the redox potential, microbial processes, and the amounts of organic matter and aeration in soil [2]. As a vital cofactor for enzymes, iron takes part in distinct processes, such as facilitating various chemical reactions, modulating protein stability, hormonal regulation, and nitrogen assimilation [1]. Iron deficiency could result in interveinal chlorosis in young leaves as the result of reduced chlorophyll content. The young leaves exhibit yellow color while the veins remain green. All these ultimately lead to the reduction of yield and quality [1,3][1][3]. In addition, other nutrients have antagonistic effects on iron uptake, which can significantly reduce the yield of the crops [4].
Iron in the rhizosphere is mainly present as Fe3+ which is not readily accessible to plants. Different plant species have evolved different strategies for iron acquisition from soil (Figure 1). Non-graminaceous plants, such as tomato and Arabidopsis, known as strategy-I plants, use a reduction-based strategy, in which plasma-membrane (PM)-localized H+-ATPases (AHAs) release the protons to increase rhizosphere acidification and promote Fe3+ solubility. Subsequently, the available ferric Fe3+ is reduced to the more soluble ferrous Fe (Fe(II)) by ferric reduction oxidases (FROs) at the apoplast [5]. The reduced ferrous ion (Fe2+) is imported into root cells by the Fe2+-regulated transporters such as the iron-regulated transporter (IRT1) [6,7][6][7]. Additionally, graminaceous plants, including rice, barley, and maize, known as strategy-II plants, use a chelation-based strategy to release phytosiderophores (PS). PS, as strong Fe chelators, are secreted into the rhizosphere with a high affinity for binding Fe (III) [8,9][8][9]. PS-Fe(III) is then taken up into root cells through the yellow stripe (YS) or yellow stripe-like (YSL) transporters [10].
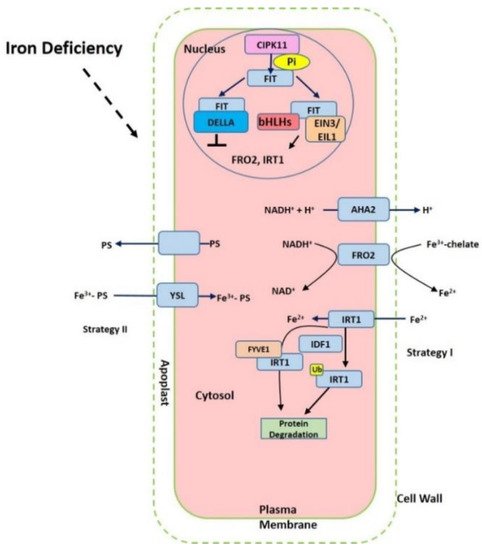
Figure 1. Summary of the iron-deficient response in plant cells. The proton ATPase AHA2, Ferric chelate reductase FRO2 (ferric reduction oxidase), Fe2+-regulated transporters iron-regulated transporter (IRT1) and FER-like iron deficiency-induced transcription factor (FIT) are activated under iron starvation, respectively. AHA2 (H+-ATPase) increases the acidification of rhizosphere to facilitate iron solubilization. FRO2 reduces ferric iron to ferrous iron that is imported into the cell via IRT1. The expression of FRO2, IRT1 can be induced via FIT interaction with other transcription factors such as bHLHs and EIN3/EIL1 but prevented with DELLA.
Iron deficiency triggers the expression of many Fe uptake-associated genes. The expression of AtAHA2 and AtAHA7, for example, are at higher levels under iron-deficient conditions, but AtAHA1 is not induced by iron deficiency [11]. Twelve PM H+-ATPases AHAs are encoded in the Arabidopsis genome [11]. AtAHA2 is primarily responsible for the of rhizosphere acidification of root hairs under iron deficiency. Loss function of AtAHA2 compromised proton extrusion capacity. AHA7 is crucial for the formation of root hairs induced by iron deficiency via mediating H+ efflux in the root hair zone. The fine-tuned regulation of root tip H+ extrusion by PM H+-ATPase is required for root hair formation. H+ efflux through PM H+-ATPase causes the acidification of the cell wall apoplast, which is crucial for the root hair initiation [11]. The loss function of AtAHA7 contributed to a decreased frequency of root hairs [11]. However, the mechanism of AHAs regulation remains unknown. Recent findings indicate that cytochrome B5 reductase 1 (CBR1) is able to activate plasma membrane-localized H+-ATPases, which is achieved by facilitating the content of unsaturated fatty acids [12]. CBR1 expression is induced under iron-deficient conditions. CBR1 localizes to endoplasmic reticulum (ER) membrane and plays an important role in electron transfer from NADH to cytochrome b5. Then the cytochrome b5 mediates the electrons transfer to fatty acids desaturase 2 (FAD2) and fatty acids desaturase 3 (FAD3), allowing for double bonds into fatty acids. FAD2 is responsible for converting oleic acid (18:1) to linoleic acid (18:2), and FAD3 contributes to the conversion of 18:2 to linolenic acid (18:3). On the other side, 20 or 50 μM of the unsaturated fatty acids 18:2 or 18:3 can strongly activate H+-ATPase [12]. Other compounds such as phenolics, organic acids, flavonoids, and flavins have also been implicated in the acidification–reduction strategy to uptake iron (Strategy I) [3,13,14,15][3][13][14][15]. These small compounds significantly promote reutilization and uptake of apoplastic iron via chelation or the reduction of iron in soil. Recently it was reported that coumarins involved in iron acquisition are secreted and essential for iron uptake under iron-limited conditions [16,17][16][17]. The plants are able to secret an array of coumarin-type compounds under different iron nutrition conditions, which facilitate Fe(III) availability [18]. The synthesis of these coumarins require Feruloyl coenzyme A 6’-hydrozylase 1 (F6’H1) enzyme [19]. ATP-BINDING CASSETTE G37 (ABCG37/PDR9) transporters contribute to the exudation of coumarins [17]. Both F6’H1 and PDR9 transcript expression are upregulated by iron deficiency [19,20][19][20].
Fe deficiency readily results in interveinal chlorosis in young leaves, ultimately reducing the yield and grain quality [43][21]. In order to tolerate iron deficiency, various physiological processes are induced in the root rhizosphere, including ferric reductase activity, the ratio of root and shoot, and photosynthesis. Also, root morphology is altered according to the local availability of iron and for optimizing iron uptake, such as increasing lateral root numbers, extra root hairs, and developing transfer cells to facilitate contact surface with soil [44][22].
3. Iron Transport Mechanism in Plants
After iron is transported to the root endodermis from epidermis via apoplastic and symplastic pathway, it needs to be transported to the above ground parts of plants through the xylem (Figure 2). The contents of organic acids, such as citrate, malate, and succinate, are elevated in xylem under iron deficient conditions [45][23]. The usage of various approaches, such as the theoretical calculations, high-pressure liquid chromatography (HPLC) coupled to electrospray time-of-flight mass spectrometry (HPLC-ESI-TOFMS) and inductively coupled plasma mass spectrometry (HPLC-ICP-MS), detects the natural Fe complex and provides evidence for the transport of iron in xylem to shoots which predominantly occurs as Fe3+-citrate complex [46,47,48,49][24][25][26][27]. The transport of citrate and iron to the xylem is mediated by ferric reductase defective 3 (FRD3) in Arabidopsis and its ortholog FRDL1 in rice, which is crucial for iron translocation [50,51][28][29]. FRD3 is present only in pericycle and cells neighboring the vascular tissue [50][28]. frd3 mutants exhibit severe Fe-deficient phenotype even under Fe-sufficient conditions. Less citrate and less Fe are contained in xylem sap of frd3 mutants as compared to wild type [50][28]. Osfrdl mutants also contain reduced citrate and Fe in the xylem resembling Fe-deficiency phenotype in frd3 mutants [52][30]. Therefore, it is tempted to speculate that graminaceous and nongraminaceous share the similar mechanism by which Fe is transported from root to shoot although the uptake strategies for iron are very different. Ferroportin1 (FPN1) is also responsible for loading iron into the xylem [44][22]. The Arabidopsis genome contains three FPN which have different subcellular localizations. FPN1, for example, is targeted to the plasma membrane, FPN2 on the vacuolar membrane and FPN3 on the chloroplast envelop [44,53,54][22][31][32]. Fe is also capable of translocation in xylem in the form of Fe-nicotianamine (NA) and Fe-MAs. NA as a non-protein amino acid is produced from S-adenosyl methionine by nicotianamine synthase (NAS) and is also the direct biochemical precursor to PS [55,56][33][34]. In rice, NA and DMA are present in xylem exudates [57,58][35][36].
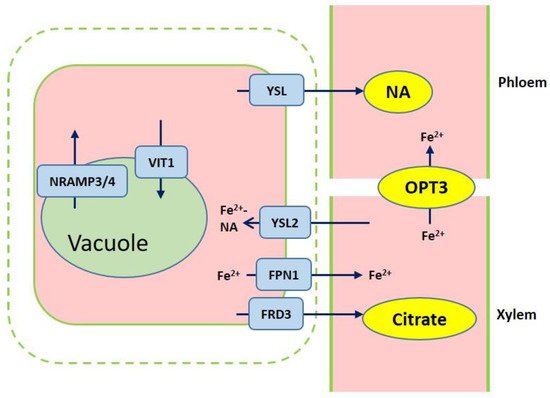
Figure 2. Overview of iron transport from roots to shoots. Ferric reductase defective 3 (FRD3) and ferroportin1 (FPN1) are responsible for importing citrate and iron into the xylem. Iron chelation with citrate or NA are translocated to shoots. Yellow stripe-like 2 (YSL2) contributes to the Fe2+-NA distribution from the xylem to neighboring cells. Iron is loaded into vacuole through VIT1, while iron efflux of vacuolar occurs via NRAMP3 and NRAMP4. OPT3 mediates the Fe transport to sink tissues via the phloem.
Once the iron reaches the leaves, it must be unloaded to leaf cells from the apoplastic space. NA and DMA are also required for the phloem-based transport [59][37]. AtYSL1, AtYSL2, and AtYSL3, as metal-NA transporters, are involved in this process, responsible for moving iron from apoplast to symplast [60,61][38][39]. These three genes are highly expressed in vascular parenchyma cells of leaves [60,61][38][39]. AtYSL2 plays a major role in regulating the lateral distribution of iron from xylem to shoot cells in Arabidopsis [54,60][32][38]. Moreover, AtYSL1 and AtYSL3 appear to transport the Fe-NA chelate from senescent leaves into the inflorescences and seeds. ysl1 and ysl3 mutants contain reduced iron content in leaves and seeds [60,62,63][38][40][41]. In rice, OsYSL2 is likely to be involved in the translocation of Fe(II)-NA to shoots and seeds [42,64][42][43]. OsYSL16 is expressed in the cells surrounding xylem and contributes to Fe(III)-MA allocation via the vascular bundle [65][44]. OsYSL18 also transports Fe(III)-DMA in reproductive organs and phloem of lamina joints [66][45]. Recent studies point to OsYSL9 which is involved in the Fe distribution in developing seeds via Fe(II)-NA and Fe(III)-DMA form [67][46]. Additionally, oligo peptide transporter 3 (OPT3) mediates the Fe transport to sink tissues via the phloem and recirculation in the roots in Arabidopsis [68][47]. Meanwhile, OPT3 is also found to take part in the control of iron movement out of the leaves to root or developing tissues in the form of iron ions rather than iron-ligand complexes [69,70][48][49]. Heat shock cognate protein B (HSCB) as a mitochondrial cochaperone participates in iron translocation from roots to shoots [71][50]. HSCB overexpression lines caused iron accumulation in roots but low iron levels in shoots; while hscb knockdown plants showed iron accumulation in shoots despite the reduced contents of iron uptake in roots [71][50].
References
- Mahender, A.; Swamy, B.P.M.; Anandan, A.; Ali, J. Tolerance of iron-deficient and -toxic soil conditions in rice. Plants 2019, 8, 31.
- Becker, M.; Asch, F. Iron toxicity in rice—Conditions and management concepts. J. Plant Nutr. Soil Sci. 2010, 168, 558–573.
- Curie, C.; Briat, J.F. Iron transport and signaling in plants. Annu. Rev. Plant Biol. 2003, 54, 183–206.
- Fageria, N.K.; Baligar, V.C.; Li, Y.C. The role of nutrient efficient plants in improving crop yields in the twenty first century. J. Plant Nutr. 2008, 31, 1121–1157.
- Yi, Y.; Guerinot, M.L. Genetic evidence that induction of root Fe(III) chelate reductase activity is necessary for iron uptake under iron deficiency. Plant J. 1996, 10, 835–844.
- Eide, D.; Broderius, M.; Fett, J.; Guerinot, M.L. A novel iron-regulated metal transporter from plants identified by functional expression in yeast. Proc. Natl. Acad. Sci. USA 1996, 93, 5624–5628.
- Vert, G.; Grotz, N.; Dédaldéchamp, F.; Gaymard, F.; Guerinot, M.L.; Briat, J.F.; Curie, C. IRT1, an Arabidopsis transporter essential for iron uptake from the soil and for plant growth. Plant Cell 2002, 14, 1223–1233.
- Takagi, S.-I. Naturally occurring iron-chelating compounds in oat- and rice-root washings. Soil Sci. Plant Nutr. 1976, 22, 423–433.
- Takagi, S.I.; Nomoto, K.; Takemoto, T. Physiological aspect of mugineic acid, a possible phytosiderophore of graminaceous plants. J. Plant Nutr. 1984, 7, 469–477.
- Curie, C.; Panaviene, Z.; Loulergue, C.; Dellaporta, S.L.; Briat, J.F.; Walker, E.L. Maize yellow stripe1 encodes a membrane protein directly involved in Fe(III) uptake. Nature 2001, 409, 346.
- Yuan, W.; Zhang, D.; Song, T.; Xu, F.; Lin, S.; Xu, W.; Li, Q.; Zhu, Y.; Liang, J.; Zhang, J. Arabidopsis plasma membrane H+-ATPase genes AHA2 and AHA7 have distinct and overlapping roles in the modulation of root tip H+ efflux in response to low-phosphorus stress. J. Exp. Bot. 2017, 68, 1731–1741.
- Oh, Y.J.; Kim, H.; Seo, S.H.; Hwang, B.G.; Chang, Y.S.; Lee, J.; Lee, D.W.; Sohn, E.J.; Lee, S.J.; Lee, Y.; et al. Cytochrome b5 reductase 1 triggers serial reactions that lead to iron uptake in plants. Mol. Plant 2016, 9, 501–513.
- Abadía, J.; López Millán, A.F.; Rombolà, A.; Abadía, A. Organic acids and Fe deficiency: A review. Plant Soil 2002, 241, 75–86.
- Jin, C.W.; You, G.Y.; He, Y.F.; Tang, C.; Wu, P.; Zheng, S.J. Iron deficiency-induced secretion of phenolics facilitates the reutilization of root apoplastic iron in red clover. Plant Physiol. 2007, 144, 278–285.
- Connorton, J.M.; Balk, J.; Rodríguez Celma, J. Iron homeostasis in plants—A brief overview. Metallomics 2017, 9, 813–823.
- Jeong, J.; Merkovich, A.; Clyne, M.; Connolly, E.L. Directing iron transport in dicots: Regulation of iron acquisition and translocation. Curr. Opin. Plant Biol. 2017, 39, 106–113.
- Clemens, S.; Weber, M. The essential role of coumarin secretion for Fe acquisition from alkaline soil. Plant Signal. Behav. 2015, 11, e1114197.
- Sisó Terraza, P.; Luis Villarroya, A.; Fourcroy, P.; Briat, J.F.; Abadía, A.; Gaymard, F.; Abadía, J.; Álvarez Fernández, A. Accumulation and secretion of coumarinolignans and other coumarins in Arabidopsis thaliana roots in response to iron deficiency at high pH. Front. Plant Sci. 2016, 7, 1711.
- Fourcroy, P.; Tissot, N.; Gaymard, F.; Briat, J.F.; Dubos, C. Facilitated Fe nutrition by phenolic compounds excreted by the Arabidopsis ABCG37/PDR9 transporter requires the IRT1/FRO2 high affinity root Fe2+ transport system. Mol. Plant 2016, 9, 485–488.
- Schmid, N.B.; Giehl, R.F.H.; Döll, S.; Mock, H.P.; Strehmel, N.; Scheel, D.; Kong, X.; Hider, R.C.; von Wirén, N. Feruloyl-CoA 6′-hydroxylase1-dependent coumarins mediate iron acquisition from alkaline substrates in Arabidopsis. Plant Physiol. 2014, 164, 160–172.
- Karim, M.R. Responses of Aerobic rice (Oryza sativa L.) to iron deficiency. J. Integr. Agric. 2012, 11, 938–945.
- Morrissey, J.; Baxter, I.R.; Lee, J.; Li, L.; Lahner, B.; Grotz, N.; Kaplan, J.; Salt, D.E.; Guerinot, M.L. The ferroportin metal efflux proteins function in iron and cobalt homeostasis in Arabidopsis. Plant Cell 2009, 21, 3326–3338.
- López-Millán, A.F.; Morales, F.; Abadía, A.; Abadía, J. Effects of iron deficiency on the composition of the leaf apoplastic fluid and xylem sap in sugar beet. Implications for iron and carbon transport. Plant Physiol. 2000, 124, 873–884.
- Durrett, T.P.; Gassmann, W.; Rogers, E.E. The FRD3-mediated efflux of citrate into the root vasculature is necessary for efficient iron translocation. Plant Physiol. 2007, 144, 197–205.
- Rellán Álvarez, R.; Giner Martínez Sierra, J.; Orduna, J.; Orera, I.; Rodríguez Castrillón, J.Á.; García Alonso, J.I.; Abadía, J.; Álvarez Fernández, A. Identification of a tri-iron(III), tri-citrate complex in the xylem sap of iron-deficient tomato resupplied with iron: New insights into plant iron long-distance transport. Plant Cell Physiol. 2009, 51, 91–102.
- Rellán-Álvarez, R.; Abadía, J.; Álvarez-Fernández, A. Formation of metal-nicotianamine complexes as affected by pH, ligand exchange with citrate and metal exchange. A study by electrospray ionization time-of-flight mass spectrometry. Rapid Commun. Mass Sp. 2008, 22, 1553–1562.
- Von Wiren, N.; Klair, S.; Bansal, S.; Briat, J.F.; Khodr, H.; Shioiri, T.; Leigh, R.A.; Hider, R.C. Nicotianamine chelates both FeIII and FeII. Implications for metal transport in plants. Plant Physiol. 1999, 119, 1107–1114.
- Green, L.S.; Rogers, E.E. FRD3 controls iron localization in Arabidopsis. Plant Physiol. 2004, 136, 2523–2531.
- Yokosho, K.; Yamaji, N.; Ma, J.F. OsFRDL1 expressed in nodes is required for distribution of iron to grains in rice. J. Exp. Bot. 2016, 67, 5485–5494.
- Yokosho, K.; Yamaji, N.; Ueno, D.; Mitani, N.; Ma, J.F. OsFRDL1 is a citrate transporter required for efficient translocation of iron in rice. Plant Physiol. 2009, 149, 297–305.
- Conte, S.; Stevenson, D.; Furner, I.; Lloyd, A. Multiple antibiotic resistance in Arabidopsis is conferred by mutations in a chloroplast-localized transport protein. Plant Physiol. 2009, 151, 559–573.
- Schaaf, G.; Häberle, J.; von Wirén, N.; Schikora, A.; Curie, C.; Vert, G.; Briat, J.-F.; Ludewig, U. A Putative function for the Arabidopsis Fe–phytosiderophore transporter homolog AtYSL2 in Fe and Zn homeostasis. Plant Cell Physiol. 2005, 46, 762–774.
- Bonneau, J.; Baumann, U.; Beasley, J.; Li, Y.; Johnson, A.A.T. Identification and molecular characterization of the nicotianamine synthase gene family in bread wheat. Plant Biotechnol. J. 2016, 14, 2228–2239.
- Inoue, H.; Higuchi, K.; Takahashi, M.; Nakanishi, H.; Mori, S.; Nishizawa, N.K. Three rice nicotianamine synthase genes, OsNAS1, OsNAS2, and OsNAS3 are expressed in cells involved in long-distance transport of iron and differentially regulated by iron. Plant J. 2003, 36, 366–381.
- Kawai, S.; Kamei, S.; Matsuda, Y.; Ando, R.; Kondo, S.; Ishizawa, A.; Alam, S. Concentrations of iron and phytosiderophores in xylem sap of iron-deficient barley plants. J. Soil Sci. Plant Nutr. 2001, 47, 265–272.
- Mori, S.; Nishizawa, N.; Hayashi, H.; Chino, M.; Yoshimura, E.; Ishihara, J. Why are young rice plants highly susceptible to iron deficiency? Plant Soil 1991, 130, 143–156.
- Curie, C.; Cassin, G.; Couch, D.; Divol, F.; Higuchi, K.; Le Jean, M.; Misson, J.; Schikora, A.; Czernic, P.; Mari, S. Metal movement within the plant: Contribution of nicotianamine and yellow stripe 1-like transporters. Ann. Bot. 2009, 103, 1–11.
- DiDonato, R.J., Jr.; Roberts, L.A.; Sanderson, T.; Eisley, R.B.; Walker, E.L. Arabidopsis Yellow Stripe-Like2 (YSL2): A metal-regulated gene encoding a plasma membrane transporter of nicotianamine–metal complexes. Plant J. 2004, 39, 403–414.
- Waters, B.M.; Chu, H.H.; DiDonato, R.J.; Roberts, L.A.; Eisley, R.B.; Lahner, B.; Salt, D.E.; Walker, E.L. Mutations in Arabidopsis Yellow Stripe-Like1 and Yellow Stripe-Like3 reveal their roles in metal ion homeostasis and loading of metal ions in seeds. Plant Physiol. 2006, 141, 1446–1458.
- Jean, M.L.; Schikora, A.; Mari, S.; Briat, J.F.; Curie, C. A loss-of-function mutation in AtYSL1 reveals its role in iron and nicotianamine seed loading. Plant J. 2005, 44, 769–782.
- Chu, H.-H.; Chiecko, J.; Punshon, T.; Lanzirotti, A.; Lahner, B.; Salt, D.E.; Walker, E.L. Successful reproduction requires the function of Arabidopsis Yellow Stripe-Like1 and Yellow Stripe-Like3 metal-nicotianamine transporters in both vegetative and reproductive structures. Plant Physiol. 2010, 154, 197–210.
- Koike, S.; Inoue, H.; Mizuno, D.; Takahashi, M.; Nakanishi, H.; Mori, S.; Nishizawa, N.K. OsYSL2 is a rice metal-nicotianamine transporter that is regulated by iron and expressed in the phloem. Plant J. 2004, 39, 415–424.
- Ishimaru, Y.; Masuda, H.; Bashir, K.; Inoue, H.; Tsukamoto, T.; Takahashi, M.; Nakanishi, H.; Aoki, N.; Hirose, T.; Ohsugi, R.; et al. Rice metal-nicotianamine transporter, OsYSL2, is required for the long-distance transport of iron and manganese. Plant J. 2010, 62, 379–390.
- Kakei, Y.; Ishimaru, Y.; Kobayashi, T.; Yamakawa, T.; Nakanishi, H.; Nishizawa, N.K. OsYSL16 plays a role in the allocation of iron. Plant Mol. Biol. 2012, 79, 583–594.
- Aoyama, T.; Kobayashi, T.; Takahashi, M.; Nagasaka, S.; Usuda, K.; Kakei, Y.; Ishimaru, Y.; Nakanishi, H.; Mori, S.; Nishizawa, N.K. OsYSL18 is a rice iron(III)-deoxymugineic acid transporter specifically expressed in reproductive organs and phloem of lamina joints. Plant Mol. Biol. 2009, 70, 681–692.
- Senoura, T.; Sakashita, E.; Kobayashi, T.; Takahashi, M.; Aung, M.; Masuda, H.; Nakanishi, H.; Nishizawa, N.K. The iron-chelate transporter OsYSL9 plays a role in iron distribution in developing rice grains. Plant Mol. Biol. 2017, 95, 375–387.
- Zhai, Z.; Gayomba, S.R.; Jung, H.I.; Vimalakumari, N.K.; Piñeros, M.; Craft, E.; Rutzke, M.A.; Danku, J.; Lahner, B.; Punshon, T.; et al. OPT3 is a phloem-specific iron transporter that is essential for systemic iron signaling and redistribution of iron and cadmium in Arabidopsis. Plant Cell 2014, 26, 2249–2264.
- Mendoza-Cózatl, D.G.; Xie, Q.; Akmakjian, G.Z.; Jobe, T.O.; Patel, A.; Stacey, M.G.; Song, L.; Demoin, D.W.; Jurisson, S.S.; Stacey, G.; et al. OPT3 is a component of the iron-signaling network between leaves and roots and misregulation of OPT3 leads to an over-accumulation of cadmium in seeds. Mol. Plant 2014, 7, 1455–1469.
- Wintz, H.; Fox, T.; Wu, Y.-Y.; Feng, V.; Chen, W.; Chang, H.-S.; Zhu, T.; Vulpe, C. Expression profiles of Arabidopsis thaliana in mineral deficiencies reveal novel transporters involved in metal homeostasis. J. Biol. Chem. 2003, 278, 47644–47653.
- Leaden, L.; Pagani, M.A.; Balparda, M.; Busi, M.V.; Gomez-Casati, D.F. Altered levels of AtHSCB disrupts iron translocation from roots to shoots. Plant Mol. Biol. 2016, 92, 613–628.
More
