Ultrasonographic elastography is a modality used to visualize the elastic properties of tissues. Technological advances in ultrasound equipment have supported the evaluation of elastography (EG) in endosonography (EUS). Currently, the usefulness of not only EUS-strain elastography (EUS-SE) but also EUS-shear wave elastography (EUS-SWE) has been reported.
- autoimmune pancreatitis
- chronic pancreatitis
- endoultrasonography
- gastro-intestinal lesion
- lymph node
- pancreatic solid lesion
- shear wave elastography
- strain elastography
- subepithelial lesion
1. Introduction
Ultrasonographic elastography, based on covering strain and shear waves, is a modality used to visualize the elastic properties of tissues. Reports in recent years have underscored the usefulness of extracorporeal ultrasonography (US) for ultrasound image enhancement for organs such as mammary, thyroid, and prostate glands. The usefulness of tissue elasticity in the gastrointestinal field for non-invasive and simple liver fibrosis diagnosis is widely acknowledged. Technological advances in ultrasound equipment have made it possible to evaluate elastography in endosonography (EUS). In 2003, EUS–strain elastography (EUS-SE) was introduced. It is reportedly useful for clinical practice, for differentiating tumors, and for diagnosing chronic pancreatitis (CP). Strain elastography (SE) was initially a qualitative examination based on color patterns, but quantitative tissue elasticity diagnosis (strain ratio (SR), histogram analysis (SH)) can be performed by image processing. Nevertheless, because tissue elasticity cannot be measured using absolute values, it can only be used as a subjective measurement. Shear wave elastography (SWE), which has been available since 2019, can objectively measure tissue elasticity using absolute values, in EUS. Reports of the usefulness of EUS–shear wave elastography (EUS-SWE) are emerging. This review presents a summary of recent advances in EUS elastography (EUS-EG) for various diseases: CP, autoimmune pancreatitis (AIP), pancreatic solid lesions (PSL), lymphadenopathy, and gastrointestinal subepithelial lesions (GI-SEL).
2. Evaluating Elastography (EG) in Endoscopic Ultrasonography
Different evaluation methods are used for elastography of different types. Different types of EG are SE including acoustic radiation force impulse (ARFI) and SWE. As explained herein, SE measures “strain,” which is correlated negatively with tissue elastic properties, whereas SWE measures shear wave velocity, which is correlated positively with true elastic properties. Today, both US and EUS can be used for SE and SWE.
Color pattern diagnosis is the qualitative evaluation method used for SE ( Figure 1 ). Generally speaking, color pattern diagnosis is used for major color tones of tumors (blue, hard; red, soft), and for heterogeneous or homogeneous color tones. Giovannini et al. first reported the elastic score: a color pattern diagnosis. The elastic score, color pattern, and heterogeneity of distribution of the elastography were classified into five types. The quantitative evaluation methods are classified into SR and SH. The former, SR, is the ratio of the target lesion strain to the peripheral tissue strain. The latter, SH, evaluates a grayscale histogram created by converting an elastography image into a gray scale of 256 tones, thereby yielding feature values. The mean value, which is one of the feature values, is reportedly correlated with the degree of pathological pancreatic fibrosis. The SH results, used along with neural network analysis (NN), are particularly valuable for differential diagnosis of pancreatic cancer from pancreatic inflammatory masses.
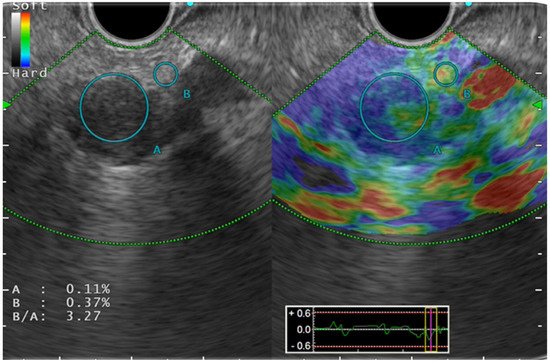
For SWE, only the quantitative evaluation method is used. Its values are measured as the shear-wave velocity (V s) and are displayed in meters per second (m/s). After Ohno et al. conducted a clinical study to validate the suitability and usefulness of EUS-SWE, they reported the success rate of pancreatic parenchymal measurement as higher than 96%. The median accuracy of measurement was 74%. For site measurement of pancreatic parenchyma hardness, the error might be caused by compression by the endoscope.
3. Endoultrasonography (EUS)-Elastography for Various Diseases
Based on those findings, EUS-EG has played an important role in CP diagnosis and pancreatic function evaluation. Moreover, the absolute values of measurements taken using EUS-SWE can enable follow-up of temporal changes of CP from an early stage.
In general, EUS-EG shows pancreatic ductal carcinoma as a heterogeneous blue color because it is harder than the surrounding pancreatic parenchyma ( Figure 2 ). Pancreatic endocrine tumors (P-NET), portrayed as blue, are homogeneous and harder than the surrounding pancreatic parenchyma. Mass-forming pancreatitis, having lower hardness than the surrounding area and heterogeneity, is shown as green. However, as chronic pancreatitis-like changes become more intense, the lesions become heterogeneous with higher hardness (blue color) than the surrounding areas, which might make it difficult to distinguish such lesions from pancreatic cancer. Numerous reports have described studies demonstrating the usefulness of EUS-EG for PSL.
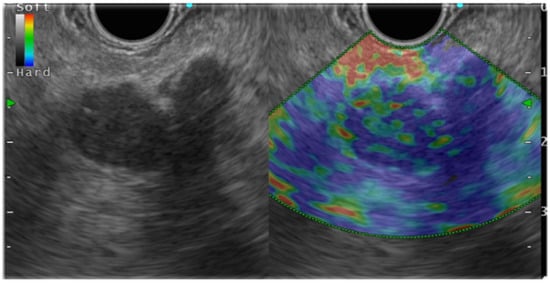
Some reports have argued that CH-EUS and EUS-EG, used in combination, can improve diagnostic performance. Chantarojanasiri et al. explained the diagnostic applicability of contrast-enhanced EUS (CH-EUS) and EUS-EG for EUS diagnosis of PSL. In fact, the positive diagnosis rates of pancreatic cancer were found to be 68% for CH-EUS, 65% for EUS-EG, and 76% for CH-EUS+EUS-EG. A meta-analysis of 17 reports of the literature indicated the pooled sensitivity and specificity for qualitative methods as 97% (95% CI, 0.95–0.99) and 67% (95% CI, 0.59–0.74), respectively. Other findings were also noteworthy: the pooled sensitivity and specificity for SH were 97% (95% CI, 0.95–0.98) and 67% (95% CI, 0.61–0.73), respectively; the pooled sensitivity and specificity for SR were 98% (95% CI, 0.96–0.99) and 62% (95% CI, 0.56–0.68), respectively; and the pooled sensitivity and specificity for CE-EUS were 90% (95% CI, 0.83–0.95) and 76% (95% CI, 0.67–0.84), respectively.
Finally, the usefulness of EUS-SWE was reported specifically in 2020. Ohno et al. reported a retrospective study comparing the respective diagnostic performances of EUS-SWE and EUS-SE for 64 PSLs. The vs. (m/s) values of PSLs were reported as 2.56 for mass-forming pancreatitis, 2.19 for pancreatic cancer, 1.58 for metastatic tumors, and 1.31 for pancreatic neuroendocrine neoplasms. Actually, vs. showed no significant difference based on the disease. The mean strain values were 74.5 for mass-forming pancreatitis, 47.3 for pancreatic neuroendocrine neoplasms, and 45.5 for pancreatic cancer. Based on a comparison between pancreatic cancer and mass-forming pancreatitis in terms of tissue elasticity, vs. was found to have no significant difference ( p = 0.5687). However, the mean strain value in pancreatic cancer cases was significantly lower: 45.4 vs. 74.5: p = 0.0007. Unexpectedly, vs. determined from EUS-SWE was found to have no significance among PSLs of different types.
4. Limitations of EUS Elastography
Major limitations of EUS-EG include intra-observer and inter-observer variation from endoscopists with varying experience. Skill and experience among these valuable professionals is crucially important to obtain reproducible results. Secondly, the pancreatic cancer detection rate of EUS-EG depends on tumor size, tumor volume, localization, and histological type. Furthermore, for EUS, some problems persist with accuracy, such as the need to rely on the heartbeat to compress the ultrasound probe.
