Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Amina Yu and Version 1 by Satya Srirama Karthik Divvela.
Atoh8 is a transcription factor that belongs to a large superfamily of transcriptional regulators called bHLH proteins. In spite of two decades of research, multiple questions regarding its molecular function and involved mechanisms remain elusive.
- Atoh8
- Math6
- cancer
- BMP
- iron-metabolism
- homeostasis
21. Atoh8
Atoh8 belongs to Group A of bHLH transcription factors that bind to the core consensus DNA sequence ‘CACCTG’ or ‘CAGCTG’ called E-Box. It is a member of the ‘Net’ family within the ‘Atonal’ superfamily [9,10][1][2]. Atoh8 was first identified in the context of neurogenesis where it was identified as another pro-neural transcription factor, like its homologs [11][3]. However, subsequent studies performed on zebrafish, chicken and mice have proved that Atoh8 is expressed ubiquitously in a temporally restricted way during embryonic development and during organogenesis.
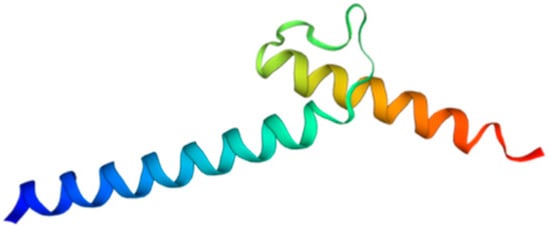
Among metazoans, Atoh8 has undergone high sequence diversification making it difficult to predict its likely functions. Yet, the bHLH domain was observed to have remained highly conserved (Figure 1). As opposed to other members of the atonal superfamily, which are encoded by a single exon, Atoh8 is exceptionally encoded by three exons (exon1, exon2 and exon3b) from zebrafish to mammals (Figure 2). In humans, Chen and his colleagues identified ‘exon3a’ in addition to exons 1, 2 and 3b (Figure 2). In addition to this, a loop acceptor and donor close to exon3a and exon3b was further identified suggesting the possibility of alternative splicing in the case of Atoh8 [12][4]. A recent study performed to investigate the role of Atoh8 in breast cancer has not only identified this novel splice variant but also attributed it to have a discrete function compared to that of the originally identified Atoh8 [13][5].
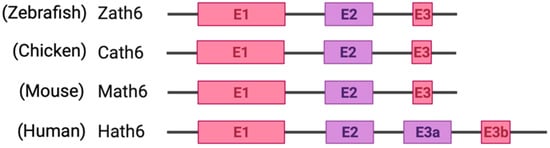
Figure 2. Schematic representation of exons and introns of Atoh8 in different orthologues. Zebrafish atonal homolog 6 (Zath6); Chicken atonal homolog 6 (Cath6); Mouse atonal homolog (Math6); Human atonal homolog 6 (Hath6).
Concerning regulatory aspects of Atoh8, the comparison of sequences upstream to Atoh8 in zebrafish, chicken, mice and humans revealed that the Atoh8 promoter has evolved from a ‘TATA box’ with a single transcriptional initiation site to the much more flexible ‘CpG islands’ with multiple transcriptional start sites that assist in fine-tuning of gene expression. This evolution in the promoter sequence also implies that Atoh8 has evolved from performing a specific function in zebrafish and chicken to multiple other functions in mice and humans, which is also in line with current literature where Atoh8 was shown to be ubiquitously expressed in mice during development [12,15][4][7]. Concerning the Atoh8 protein, in addition to the highly conserved bHLH domain, Atoh8 also possesses a proline-rich domain and serine-rich domain which are located in the N-terminus. The proline-rich domain in proteins such as p53 and Hex was implicated to repress transcription directly or by acting as co-repressors [16,17][8][9]. Although the proline-rich region of Atoh8 was attributed to its negative regulatory function, conclusive evidence of such repressive activity by the proline-rich domain is still missing. The serine-rich domain is liable for phosphorylation and dephosphorylation within proteins. The phosphorylation state of serine-rich domains is perceived to modulate gene expression temporally in the case of viral genes [18][10]. Likewise, the serine-rich domain of Atoh8 was identified to interact with calcineurin which is downstream of calcium signaling. The serine/threonine phosphatase Calcineurin was proposed to dephosphorylate Atoh8 thereby promoting its translocation into the nucleus where it exerts its function as a transcription factor [19][11].
2. Atoh8 in Disease
2.1. Atoh8 in Cancer
Given the ubiquitous expression and multiple regulatory roles of Atoh8 during embryonic development, it is not at all surprising to see its involvement in cancer and several other disorders. The high-grade gliomas are diagnosed on the basis of different molecular markers. The 1p19q codeletion is one characteristic marker for oligodendroglioma. In contrast, the epidermal growth factor receptor (EGFR) amplification is seen in glioblastoma. In 2008, a study performed to understand the two mutually exclusive groups of gliomas carrying ‘1p19q codeletion’ and ‘EGFR amplification’, identified Atoh8 as one of the multiple differentially regulated genes. Higher expression of Atoh8 was found to be correlated with the pro-neural group of gliomas particularly oligodendroglioma with ‘1p19q codeletion’ and lower expression of Atoh8 was found to be correlated with a proliferative and mesenchymal group of gliomas with ‘EGFR amplification’. Likewise, another study which was performed to ascertain the copy number variations in glioblastomas has also revealed aberrant expression of Atoh8 further suggesting it as a potential candidate involved in carcinogenesis [50,51][12][13]. Following this, another study which was investigating the effect of retinoic acid on glioblastoma stem cell-like cells also showed an increase in the expression of Atoh8 following treatment with retinoic acid which was found to be correlated with differentiation of the glioblastoma stem cell-like population [52][14]. In recent years, Atoh8 has been further studied in other types of cancers. In hepatitis B virus-associated hepatocellular carcinomas (HCC), reduced levels of Atoh8 were observed to be correlated with loss of tumor differentiation. Confirming the same, analysis of additional hepatic carcinoma cell lines such as HepG2, PLC8024 and CRL8064 also revealed a correlation between reduced Atoh8 expression and increased CD133 positive population. Additionally, the authors have also evaluated the effect of overexpression of Atoh8 in the hepatic carcinoma cell lines, which resulted in the reduction of the proliferation along with reduced invasive and migratory abilities of those cells. Lastly, overexpression of Atoh8 was also shown to reduce tumor formation and increase chemosensitivity in these cells. Most importantly, ithis study was showed that Atoh8 could repress core regulators of pluripotency such as Oct4 and Nanog by binding to their E-Box sequences [22,53][15][16].
In nasopharyngeal carcinomas (NPC), similar to the findings in hepatic carcinomas, the inhibition of Atoh8 was shown to enhance the malignant phenotype, whereas its transgenic expression was shown to reverse the phenotype. In this study, the author was identified the silencing of Atoh8 in tumor cells as an epigenetic mechanism induced by the tumorigenic LMP1 (Latent Membrane Protein-1). In tumor cells, the occupancy of active H3K4me3 of the Atoh8 promoter sequence was found to be replaced by a repressive H3K27me3 mark. Lastly, this studyit was further described Atoh8 as a regulator of epithelial-mesenchymal transition (EMT) because of its correlation with EMT markers [54][17].
In colorectal cancer (CRC), higher expression of Atoh8 was found to correlate with the poor prognosis of CRC patients. The knockdown of Atoh8 in CRC cells was shown to reduce proliferation with an accumulation of cells in the S phase of the cell cycle. Additionally, the depletion of Atoh8 in CRC cells was observed to increase apoptosis with no apparent changes to the migratory ability of the cells which is in clear contrast to the findings observed in HCC and NPC [55][18]. Another study, which used colorectal cancer cells as mimics of circulating tumor cells (m-CTCs), showed Atoh8 as a mechanosensor following their exposure to laminar shear stress. This study indicated that activation of Atoh8 in m-CTCs increased their plasticity and intravascular survival suggesting a promoting effect of Atoh8 [56][19].
In breast cancer, a situdy was performed to identify the enriched tissue-specific transcription factors which connected Atoh8 to the dysregulated transcriptional regulatory network which affected the proliferation, differentiation, cell adhesion and metastasis [57][20]. Interestingly, a most recent study has identified a novel isoform of Atoh8 called Atoh8-V1 in breast cancer, it was shown to be a negative prognostic marker with a high expression. Atoh8-V1 was further shown to bind and activate the RhoC promoter which in turn promotes metastasis in breast cancer. Similarly, downregulation of Atoh8-V1 was shown to correlate with reduced metastasis [13][5]. Given this new transcriptional variant, it would be interesting to see if the contradictory reports that are mentioned in the case of different cancers result from divergent functions of Atoh8 or because of the existence of more transcriptional variants.
2.2. Atoh8 in Cellular Homeostasis
So far, Atoh8 has been implicated to be involved in cellular stress response, hypoxia response, and iron metabolism. Atoh8 was identified to be activated by shear stress during endothelial cell differentiation. It was further reported that overexpression of Atoh8 mimicked shear-stress treatment attributing a major role to Atoh8 in stress management. In addition to shear stress, IFN-γ, TNF-α and oxidative stress were shown to act upstream and activate Atoh8 expression in endothelial cells. Furthermore, eNOS which plays a major role in endothelial cell protection, nitric oxide production was shown to be positively regulated, at the same time as a direct target of Atoh8 [21,47][23][24]. The phenotype observed in Atoh8 knockout mice which was similar to pulmonary arterial hypertension together with the reduced levels of the eNOS following knock-down or absence of Atoh8 correlates with the phenotype of defective placental development observed in Atoh8 knockout mice (Table 1) [21,43,48][23][25][26]. In addition to this, it would also be of great interest to check if Atoh8-associated stress response is restricted to the endothelial cells or if it could perform a similar function in other cell types as it is ubiquitously expressed.
Table 1. List of Atoh8 mutant mice and observed phenotypes.
| Deletion of Genomic Region | Reported Phenotype | Mouse Strain | References | |
|---|---|---|---|---|
| Exon 1 and 2 | Early embryonic lethality | C57BL/6 | [30] | [27] |
| Exon 1 | Normal | Mixed background C57BL/6 and SV/129 | [39] | [28] |
| Exon 1 | Defective placenta development | C57BL/6NJ | [43] | [25] |
| Exon 1 | Pulmonary arterial hypertension and delayed retinal angiogenesis | C57BL/6 | [48] | [26] |
| Exon 1 | Hearing loss | Mixed background C57BL/6J and 129S6 | [60] | [29] |
Atoh8 was identified as a downstream factor for activating transcription factor 4 (ATF4) in pancreatic ß-cells. ATF4 is a transcriptional regulator for integrated stress response (ISR) and unfolded proteins. Severe diabetes is led by dysregulation of ISR. Thereby ATF4 senses endoplasmic reticulum stress in cellular pathologies and is responsible for the maintenance of homeostasis in the endoplasmic reticulum. The ISR enhancer Sephin1 increased the ATF4 expression in the pancreatic islets and increased the insulin secretion [61][30].
Lately, Atoh8 was also shown to regulate hypoxic response. A study by Morikawa’s group showed that Atoh8 binds hypoxia-inducible factor 2α (HIF-2α) and decreases its abundance leading to the attenuation of the hypoxic response. It was further shown that the Alk1/Smad/Atoh8 axis acts as a regulator of the hypoxic response. Interestingly, analysis performed on HPAECs showed the HLH domain of Atoh8 to be crucial for hypoxic response with no functional role of the basic domain [48][26]. Recently, another study performed on hypoxia adaptation of Tibetan pigs also showed Atoh8 as a potential regulator of hypoxia, which has the potential to modulate TGF-ß and PI3K-AKT signaling [62][31].
In addition to stress and hypoxia regulation, Atoh8 was also shown to regulate metabolism, especially iron metabolism. Atoh8 was observed to regulate hepcidin which prevents the overloading of iron in the body. In hepatocytes, BMP6 was shown to activate Atoh8 (Figure 3), which further binds to the promoter of hepcidin and promotes hepcidin production, ultimately regulating the uptake of iron by the duodenal epithelium [63,64][32][33]. Despite the existence of multiple Atoh8 knockout mouse models, no phenotype concerning iron metabolism or liver has been reported so far.
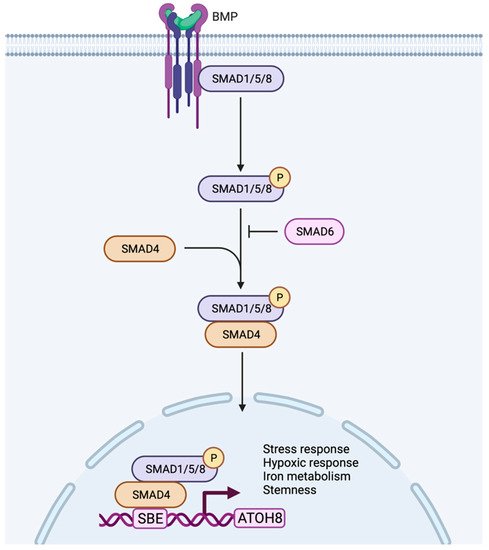
Figure 3. Working model of Atoh8 activation by canonical BMP signaling pathway. In the canonical pathway, BMPs transduce their signal by assembling type I or type II receptors to form a hetero-tetrameric complex. The assembly of receptors initiates transphosphorylation of type I receptors by type II receptors which further phosphorylates and activates R-Smads (Smad 1/5/8). The activated Smad1/5/8 interacts with Co-Smad (Smad4) to form a complex that further translocate into the nucleus to bind to the Smad Binding Element (SBE) of Atoh8 to initiate its transcription. Based on the current literature, the activated Atoh8 can be considered to regulate stress response, hypoxic response, iron metabolism and stemness of the cells.
References
- Simionato, E.; Ledent, V.; Richards, G.; Thomas-Chollier, M.; Kerner, P.; Coornaert, D.; Degnan, B.M.; Vervoort, M. Origin and Diversification of the Basic Helix-Loop-Helix Gene Family in Metazoans: Insights from Comparative Genomics. BMC Evol. Biol. 2007, 7, 33.
- Wang, Y.; Chen, K.; Yao, Q.; Zheng, X.; Yang, Z. Phylogenetic Analysis of Zebrafish Basic Helix-Loop-Helix Transcription Factors. J. Mol. Evol. 2009, 68, 629–640.
- Inoue, C.; Bae, S.; Takatsuka, K.; Inoue, T.; Bessho, Y.; Kageyama, R. Math6, a BHLH Gene Expressed in the Developing Nervous System, Regulates Neuronal versus Glial Differentiation. Genes Cells 2001, 6, 977–986.
- Chen, J.; Dai, F.; Balakrishnan-Renuka, A.; Leese, F.; Schempp, W.; Schaller, F.; Hoffmann, M.M.; Morosan-Puopolo, G.; Yusuf, F.; Bisschoff, I.; et al. Diversification and Molecular Evolution of ATOH8, a Gene Encoding a BHLH Transcription Factor. PLoS ONE 2011, 6, e23005.
- Xu, M.; Huang, S.; Dong, X.; Chen, Y.; Li, M.; Shi, W.; Wang, G.; Huang, C.; Wang, Q.; Liu, Y.; et al. A Novel Isoform of ATOH8 Promotes the Metastasis of Breast Cancer by Regulating RhoC. J. Mol. Cell Biol. 2020, 13, 59–71.
- Bienert, S.; Waterhouse, A.; de Beer, T.A.P.; Tauriello, G.; Studer, G.; Bordoli, L.; Schwede, T. The SWISS-MODEL Repository—New Features and Functionality. Nucleic Acids Res. 2017, 45, D313–D319.
- Wang, B.; Balakrishnan-Renuka, A.; Napirei, M.; Theiss, C.; Brand-Saberi, B. Spatiotemporal Expression of Math6 during Mouse Embryonic Development. Histochem. Cell Biol. 2015, 143, 575–582.
- Swingler, T.E.; Bess, K.L.; Yao, J.; Stifani, S.; Jayaraman, P.-S. The Proline-Rich Homeodomain Protein Recruits Members of the Groucho/Transducin-like Enhancer of Split Protein Family to Co-Repress Transcription in Hematopoietic Cells. J. Biol. Chem. 2004, 279, 34938–34947.
- Venot, C.; Maratrat, M.; Dureuil, C.; Conseiller, E.; Bracco, L.; Debussche, L. The Requirement for the P53 Proline-Rich Functional Domain for Mediation of Apoptosis Is Correlated with Specific PIG3 Gene Transactivation and with Transcriptional Repression. EMBO J. 1998, 17, 4668–4679.
- Chen, C.; Agnès, F.; Gélinas, C. Mapping of a Serine-Rich Domain Essential for the Transcriptional, Antiapoptotic, and Transforming Activities of the v-Rel Oncoprotein. Mol. Cell Biol. 2001, 21, 7115.
- Chen, J.; Balakrishnan-Renuka, A.; Hagemann, N.; Theiss, C.; Chankiewitz, V.; Chen, J.; Pu, Q.; Erdmann, K.S.; Brand-Saberi, B. A Novel Interaction between ATOH8 and PPP3CB. Histochem. Cell Biol. 2016, 145, 5–16.
- Ducray, F.; Idbaih, A.; de Reyniès, A.; Bièche, I.; Thillet, J.; Mokhtari, K.; Lair, S.; Marie, Y.; Paris, S.; Vidaud, M.; et al. Anaplastic Oligodendrogliomas with 1p19q Codeletion Have a Proneural Gene Expression Profile. Mol. Cancer 2008, 7, 41.
- Freire, P.; Vilela, M.; Deus, H.; Kim, Y.-W.; Koul, D.; Colman, H.; Aldape, K.D.; Bogler, O.; Yung, A.W.; Coombes, K.; et al. Exploratory Analysis of the Copy Number Alterations in Glioblastoma Multiforme. PLoS ONE 2008, 3, e4076.
- Ying, M.; Wang, S.; Sang, Y.; Sun, P.; Lal, B.; Goodwin, C.; Guerrero-Cazares, H.; Quinones-Hinojosa, A.; Laterra, J.; Xia, S. Regulation of Glioblastoma Stem Cells by Retinoic Acid: Role for Notch Pathway Inhibition. Oncogene 2011, 30, 3454–3467.
- Song, Y.; Pan, G.; Chen, L.; Ma, S.; Zeng, T.; Chan, T.; Li, L.; Lian, Q.; Chow, R.; Cai, X.; et al. Loss of ATOH8 Increases Stem Cell Features of Hepatocellular Carcinoma Cells. Gastroenterology 2015, 149, 1068–1081.e5.
- Zhao, F.; Yu, J. Unearthing a Novel Tumor Suppressor Function of ATOH8 in Hepatocellular Carcinoma: Role in Acquisition of Cancer Stem Cell-like Features. Transl. Cancer Res. 2016, 5, S91–S94.
- Wang, Z.; Xie, J.; Yan, M.; Wang, J.; Wang, X.; Zhang, J.; Zhang, Y.; Li, P.; Lei, X.; Huang, Q.; et al. Downregulation of ATOH8 Induced by EBV-Encoded LMP1 Contributes to the Malignant Phenotype of Nasopharyngeal Carcinoma. Oncotarget 2015, 7, 26765–26779.
- Ye, M.; He, Y.; Lin, H.; Yang, S.; Zhou, Y.; Zhou, L.; Zhong, J.; Lu, G.; Zheng, J.; Xue, Z.-X.; et al. High Expression of Atonal Homolog 8 Predicts a Poor Clinical Outcome in Patients with Colorectal Cancer and Contributes to Tumor Progression. Oncol. Rep. 2017, 37, 2955–2963.
- Huang, Q.; Li, S.; Hu, X.; Sun, M.; Wu, Q.; Dai, H.; Tan, Y.; Sun, F.; Wang, C.; Rong, X.; et al. Shear Stress Activates ATOH8 via Autocrine VEGF Promoting Glycolysis Dependent-Survival of Colorectal Cancer Cells in the Circulation. J. Exp. Clin. Cancer Res. 2020, 39, 25.
- Li, W.-X.; He, K.; Tang, L.; Dai, S.-X.; Li, G.-H.; Lv, W.-W.; Guo, Y.-C.; An, S.-Q.; Wu, G.-Y.; Liu, D.; et al. Comprehensive Tissue-Specific Gene Set Enrichment Analysis and Transcription Factor Analysis of Breast Cancer by Integrating 14 Gene Expression Datasets. Oncotarget 2017, 8, 6775–6786.
- Ke, X.-S.; Qu, Y.; Cheng, Y.; Li, W.-C.; Rotter, V.; Øyan, A.; Kalland, K.-H. Global Profiling of Histone and DNA Methylation Reveals Epigenetic-Based Regulation of Gene Expression during Epithelial to Mesenchymal Transition in Prostate Cells. BMC Genom. 2010, 11, 669.
- Wan, F.; Zhu, Y.; Han, C.; Xu, Q.; Wu, J.; Dai, B.; Zhang, H.; Shi, G.; Gu, W.; Ye, D. Identification and Validation of an Eight-Gene Expression Signature for Predicting High Fuhrman Grade Renal Cell Carcinoma. Int. J. Cancer 2017, 140, 1199–1208.
- Fang, F.; Wasserman, S.M.; Torres-Vazquez, J.; Weinstein, B.; Cao, F.; Li, Z.; Wilson, K.D.; Yue, W.; Wu, J.C.; Xie, X.; et al. The Role of Hath6, a Newly Identified Shear-Stress-Responsive Transcription Factor, in Endothelial Cell Differentiation and Function. J. Cell Sci. 2014, 127, 1428–1440.
- Wasserman, S.M.; Mehraban, F.; Komuves, L.G.; Yang, R.-B.; Tomlinson, J.E.; Zhang, Y.; Spriggs, F.; Topper, J.N. Gene Expression Profile of Human Endothelial Cells Exposed to Sustained Fluid Shear Stress. Physiol. Genom. 2002, 12, 13–23.
- Böing, M.; Brand-Saberi, B.; Napirei, M. Murine Transcription Factor Math6 Is a Regulator of Placenta Development. Sci. Rep. 2018, 8, 14997.
- Morikawa, M.; Mitani, Y.; Holmborn, K.; Kato, T.; Koinuma, D.; Maruyama, J.; Vasilaki, E.; Sawada, H.; Kobayashi, M.; Ozawa, T.; et al. The ALK-1/SMAD/ATOH8 Axis Attenuates Hypoxic Responses and Protects against the Development of Pulmonary Arterial Hypertension. Sci. Signal. 2019, 12, eaay4430.
- Lynn, F.C.; Sanchez, L.; Gomis, R.; German, M.S.; Gasa, R. Identification of the BHLH Factor Math6 as a Novel Component of the Embryonic Pancreas Transcriptional Network. PLoS ONE 2008, 3, e2430.
- Rawnsley, D.R.; Xiao, J.; Lee, J.S.; Liu, X.; Mericko-Ishizuka, P.; Kumar, V.; He, J.; Basu, A.; Lu, M.; Lynn, F.C.; et al. The Transcription Factor Atonal Homolog 8 Regulates Gata4 and Friend of Gata-2 during Vertebrate Development. J. Biol. Chem. 2013, 288, 24429–24440.
- Tang, Q.; Xie, M.; Zhang, Y.; Xue, R.; Zhu, X.; Yang, H. Targeted Deletion of Atoh8 Results in Severe Hearing Loss in Mice. Genesis 2021, 59, e23442.
- Kitakaze, K.; Oyadomari, M.; Zhang, J.; Hamada, Y.; Takenouchi, Y.; Tsuboi, K.; Inagaki, M.; Tachikawa, M.; Fujitani, Y.; Okamoto, Y.; et al. ATF4-Mediated Transcriptional Regulation Protects against β-Cell Loss during Endoplasmic Reticulum Stress in a Mouse Model. Mol. Metab. 2021, 54, 101338.
- Wang, T.; Guo, Y.; Liu, S.; Zhang, C.; Cui, T.; Ding, K.; Wang, P.; Wang, X.; Wang, Z. KLF4, a Key Regulator of a Transitive Triplet, Acts on the TGF-β Signaling Pathway and Contributes to High-Altitude Adaptation of Tibetan Pigs. Front. Genet. 2021, 12, 628192.
- Kautz, L.; Meynard, D.; Monnier, A.; Darnaud, V.; Bouvet, R.; Wang, R.-H.; Deng, C.; Vaulont, S.; Mosser, J.; Coppin, H.; et al. Iron Regulates Phosphorylation of Smad1/5/8 and Gene Expression of Bmp6, Smad7, Id1, and Atoh8 in the Mouse Liver. Blood 2008, 112, 1503–1509.
- Patel, N.; Varghese, J.; Masaratana, P.; Latunde-Dada, G.O.; Jacob, M.; Simpson, R.J.; McKie, A.T. The Transcription Factor ATOH8 Is Regulated by Erythropoietic Activity and Regulates HAMP Transcription and Cellular PSMAD1,5,8 Levels. British J. Haematol. 2014, 164, 586–596.
More