LED lighting is increasingly applied to increase yield and quality of greenhouse produced crops, especially tomatoes. Tomatoes cannot be stored at cold temperatures due to chilling injury that manifests as quick quality deterioration during shelf life.
- tomato fruit
- blue light
- chilling injury
- postharvest
1. Introduction
2. Effect of Light Treatments on CI in R and MG Fruit
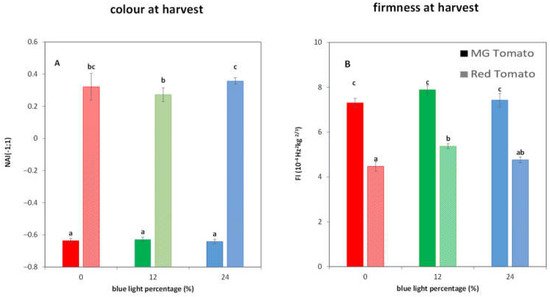
3.2. Light Treatments Affect the Colour and Firmness at Harvest in R Tomatoes
3. Light Treatments Affect the Colour and Firmness at Harvest in R Tomatoes
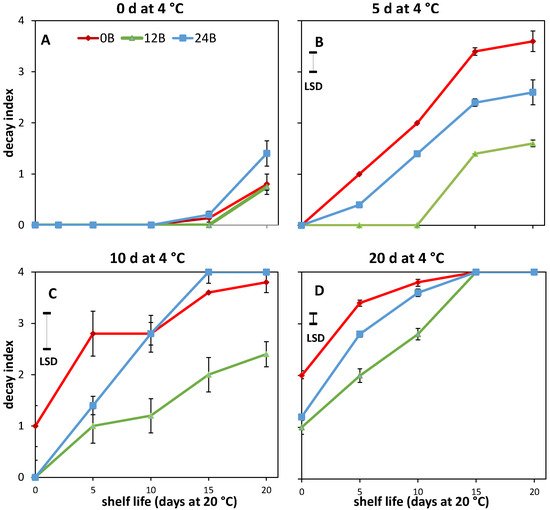
34. Effect of Light Treatments and Cold Storage on Coloration and Softening of MG Fruit
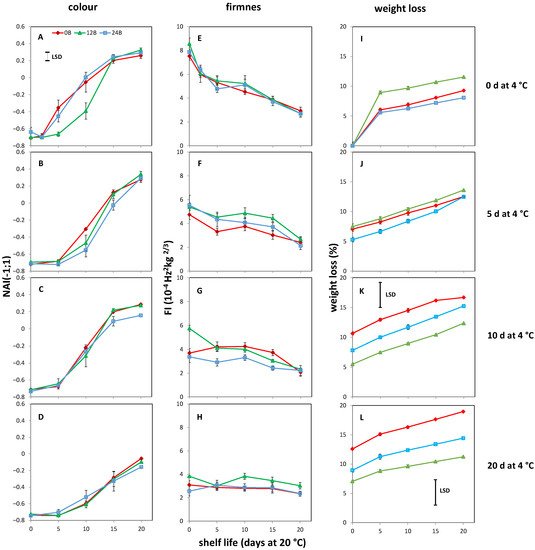
45. Cold-Stored R Tomatoes Show Colour and Firmness Loss
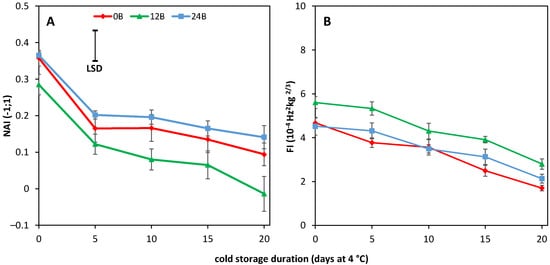
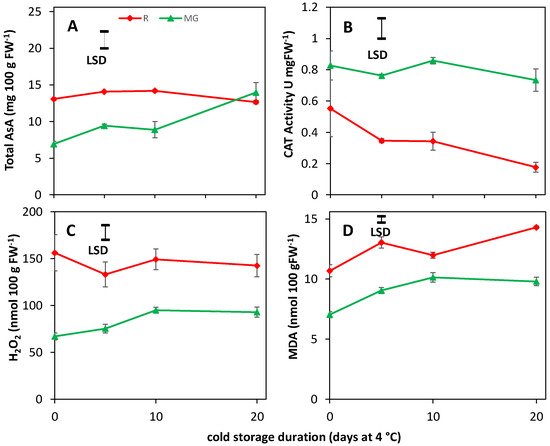
56. AsA, CAT Activity, H2O2 and MDA Content Are Unaffected by BL Treatments
67. Summary
The chilling tolerance of red harvested tomato fruit was improved only by moderate blue light addition (12%) on top of a red background during cultivation. This improved cold tolerance for the R fruit was not due to differences in CAT activity, total ascorbic acid, H2O2 and MDA levels, but due to a lower red color at harvest and faster discoloration during cold storage. The red color measurement, measured by remittance spectroscopy, is closely related to the lycopene concentration. It is hypothesized that the lower lycopene content of the R fruit cultivated with moderate blue light levels allows for more lycopene loss during cold storage, thereby creating a higher cold tolerance.
References
- Albornoz, K.; Cantwell, M.I.; Zhang, L.; Beckles, D.M. Integrative analysis of postharvest chilling injury in cherry tomato fruit reveals contrapuntal spatio-temporal responses to ripening and cold stress. Sci. Rep. 2019, 9, 2759.
- Loayza, F.E.; Brecht, J.K.; Simonne, A.H.; Plotto, A.; Baldwin, E.A.; Bai, J.; Lon-Kan, E. A brief hot-water treatment alleviates chilling injury symptoms in fresh tomatoes. J. Sci. Food Agric. 2021, 101, 54–64.
- Biswas, P.; East, A.R.; Brecht, J.K.; Hewett, E.W.; Heyes, J.A. Intermittent warming during low temperature storage reduces tomato chilling injury. Postharvest Biol. Technol. 2012, 74, 71–78.
- Farneti, B.; Schouten, R.E.; Woltering, E.J. Low temperature-induced lycopene degradation in red ripe tomato evaluated by remittance spectroscopy. Postharvest Biol. Technol. 2012, 73, 22–27.
- Sevillano, L.; Sanchez-Ballesta, M.T.; Romojaro, F.; Flores, F.B. Physiological, hormonal and molecular mechanisms regulating chilling injury in horticultural species. Postharvest technologies applied to reduce its impact. J. Sci. Food Agric. 2009, 89, 555–573.
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930.
- Hodges, D.M.; Lester, G.E.; Munro, K.D.; Toivonen, P.M. Oxidative stress: Importance for postharvest quality. Hortscience 2004, 39, 924–929.
- Zhao, R.; Sheng, J.; Lv, S.; Zheng, Y.; Zhang, J.; Yu, M.; Shen, L. Nitric oxide participates in the regulation of LeCBF1 gene expression and improves cold tolerance in harvested tomato fruit. Postharvest Biol. Technol. 2011, 62, 121–126.
- Biswas, P.; East, A.R.; Hewett, E.W.; Heyes, J.A. Chilling injury in tomato fruit. Hortic. Rev. 2016, 44, 229–278.
- Jackman, R.L.; Gibson, H.J.; Stanley, D.W. Effects of chilling on tomato fruit texture. Physiol. Plant. 1992, 86, 600–608.
- Marangoni, A.G.; Jackman, R.L.; Stanley, D.W. Chilling-associated softening of tomato fruit is related to increased pectinmethylesterase activity. J. Food Sci. 1995, 60, 1277–1281.
- Rugkong, A.; McQuinn, R.; Giovannoni, J.J.; Rose, J.K.; Watkins, C.B. Expression of ripening-related genes in cold-stored tomato fruit. Postharvest Biol. Technol. 2011, 61, 1–14.
- Aghdam, M.S.; Bodbodak, S. Postharvest heat treatment for mitigation of chilling injury in fruits and vegetables. Food Bioprocess Technol. 2014, 7, 37–53.
- Imahori, Y.; Bai, J.; Baldwin, E. Antioxidative responses of ripe tomato fruit to postharvest chilling and heating treatments. Sci. Hortic. 2016, 198, 398–406.
- Malacrida, C.; Valle, E.M.; Boggio, S.B. Postharvest chilling induces oxidative stress response in the dwarf tomato cultivar Micro-Tom. Physiol. Plant. 2006, 127, 10–18.
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611.
- Toor, R.K.; Savage, G.P. Antioxidant activity in different fractions of tomatoes. Food Res. Int. 2005, 38, 487–494.
- Foyer, C.H.; Noctor, G. Ascorbate and Glutathione: The heart of the redox hub. Plant Physiol. 2011, 155, 2–18.
- Schouten, R.E.; Huijben, T.P.; Tijskens, L.M.M.; van Kooten, O. Modelling quality attributes of truss tomatoes: Linking colour and firmness maturity. Postharvest Biol. Technol. 2007, 45, 298–306.
- Heymann, T.; Heinz, P.; Glomb, M.A. Lycopene Inhibits the Isomerization of β-Carotene during Quenching of Singlet Oxygen and Free Radicals. J. Agric. Food Chem. 2015, 63, 3279–3287.
- Stahl, W.; Sies, H. Antioxidant activity of carotenoids. Mol. Asp. Med. 2003, 24, 345–351.
- Brandt, S.; Pék, Z.; Barna, É.; Lugasi, A.; Helyes, L. Lycopene content and colour of ripening tomatoes as affected by environmental conditions. J. Sci. Food Agric. 2006, 86, 568–572.
- Neta-Sharir, I.; Isaacson, T.; Lurie, S.; Weiss, D. Dual role for tomato heat shock protein 21: Protecting photosystem ii from oxidative stress and promoting color changes during fruit maturation. Plant Cell 2005, 17, 1829–1838.
- Hernández, V.; Hellín, P.; Fenoll, J.; Flores, P. Increased temperature produces changes in the bioactive composition of tomato, depending on its developmental stage. J. Agric. Food Chem. 2015, 63, 2378–2382.
- Lu, J.; Nawaz, M.A.; Wei, N.; Cheng, F.; Bie, Z. Suboptimal temperature acclimation enhances chilling tolerance by improving photosynthetic adaptability and osmoregulation ability in watermelon. Hortic. Plant J. 2020, 6, 49–60.
- Jiang, M.Y.; Zhang, J.H. Water stress-induced abscisic acid accumulation triggers the increased generation of reactive oxygenspecies and up-regulates the activities of antioxidant enzymes inmaize leaves. J. Expt. Bot. 2002, 379, 2401–2410.
- Yacoubi, I.; Hamdi, K.; Fourquet, P.; Bignon, C.; Longhi, S. Structural and functional characterization of the aba-water deficit stress domain from wheat and barley: An intrinsically disordered domain behind the versatile functions of the plant abscissic acid, stress and ripening protein family. Int. J. Mol. Sci. 2021, 22, 2314.
- Singh, A.P.; Mani, B.; Giri, J. OsJAZ9 is involved in water-deficit stress tolerance by regulating leaf width and stomatal density in rice. Plant Physiol. Biochem. 2021, 162, 161–170.
- Hanin, M.; Brini, F.; Ebel, C.; Toda, Y.; Takeda, S.; Masmoudi, K. Plant dehydrins and stress tolerance: Versatile proteins for complex mechanisms. Plant Signal. Behav. 2011, 6, 1503–1509.
- Yu, Z.; Wang, X.; Zhang, L. Structural and functional dynamics of dehydrins: A plant protector protein under abiotic stress. Int. J. Mol. Sci. 2018, 19, 3420.
- Hara, M.; Terashima, S.; Fukaya, T.; Kuboi, T. Enhancement of cold tolerance and inhibition of lipid peroxidation by citrus dehydrin in transgenic tobacco. Planta 2003, 217, 290–298.
- Zhuo, C.; Liang, L.; Zhao, Y.; Guo, Z.; Lu, S. A cold responsive ethylene responsive factor from Medicago falcata confers cold tolerance by up-regulation of polyamine turnover, antioxidant protection, and proline accumulation. Plant Cell Environ. 2017, 41, 2021–2032.
- Affandi, F.Y.; Verdonk, J.C.; Ouzounis, T.; Ji, Y.; Woltering, E.J.; Schouten, R.E. Far-red light during cultivation induces postharvest cold tolerance in tomato fruit. Postharvest Biol. Technol. 2020, 159, 111019.
- Shi, Y.; Huang, J.; Sun, T.; Wang, X.; Zhu, C.; Ai, Y.; Gu, H. The precise regulation of differentCORgenes by individual CBF transcription factors inArabidopsis thaliana. J. Integr. Plant Biol. 2017, 59, 118–133.
- Rihan, H.Z.; Al-Issawi, M.; Fuller, M.P. Advances in physiological and molecular aspects of plant cold tolerance. J. Plant Interact. 2017, 12, 143–157.
- Chen, B.; Feder, M.E.; Kang, L. Evolution of heat-shock protein expression underlying adaptive responses to envi-ronmental stress. Mol. Ecol. 2018, 27, 3040–3054.
