You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Vivi Li and Version 1 by Laura Graciela Mereles Ceuppens.
The “Kurugua” (Sicana odorifera) is a native fruit that demonstrates attractive nutritional, coloring, flavoring, and antioxidant properties. The main by-products from the processing and consumption of kurugua fruit are epicarp and seeds.
- Sicana odorifera
- seeds
- proximate composition
- minerals
- antioxidant activity
- hepatoprotective
- fatty acids
- biowaste
- by-products
1. Introduction
Current global challenges such as food safety, climate change, poverty, and health have a direct impact on the realization of the right to adequate food.
Each challenge is negatively affected by food loss and waste, and developing sustainable global consumption and production systems is necessary [1]. Food by-products management has been recognized by the circular economy as one of the principal keys to reducing environmental and economic problems [2]. The recovery of bioactive molecules from the bio-residues or industrial by-products of fruits and vegetables has potential uses in the industrial sector. The processed fruits’ waste can be re-used and can lead to high-added-value products such as functional food ingredients, food coloring, novel pharmaceuticals for alternative therapies, and disease prevention [3].
The interest of the food and cosmetic industries for products from medicinal plants has increased, and it is necessary to broaden the investigation of natural sources, including fruit waste such as seeds [4]. The literature on value addition to fruit-derived waste is diverse. Overall, the extraction of bioactive compounds from fruit processing waste and the application of green methods for the valorization of these sources opens new avenues for food, chemical, and pharmaceutical industries, which have high potential, especially where availability of waste from fruit processing is abundant [5]. This current trend has led researchers to explore several methods to recycle waste for manufacturing new products, emphasizing green chemistry and greener processes. Melon by-products have been used as new feedstock for proteins’ recovery, employing biological precipitation; the cucumisin was separated from these by-products with carrageenan, an environmentally friendly process for the industries, which avoids solvents [2]. A novel bio-refinery approach would seek to produce a wider range of valuable chemicals from fruit processing waste. For instance, the residue from most of extraction processes could further be a renewable source of biofuels, and polyphenols may be useful as food products and pharmaceuticals preservatives [5]. Quercetin and quercetin-3-glycoside are being isolated from the fruit seed waste of papaya seeds. Grape seeds are rich in polyphenols, resveratrol, quercetin, and other flavonoids, which are confirmed to impart cardiovascular protective effects [6]. Natural flavoring agents such as limonene, pectin extraction from fruit peels, and the growing demand for natural products in the food and beverage industry show the scope of technological progress in this sector [5].
The year 2021 has been declared as the International Year of Fruits and Vegetables, encouraging populations to increase their consumption, seeking for the reduction of waste within the framework of Sustainable Food and Food Security, and valuing the potential of by-products, such as the seeds, not used in the industrial processing of fruits [7]. It has been reported that the processing of fruits or vegetables belonging to genera of Cucurbitaceae such as Cucumis (melon), Cucurbita (pumpkin), and Citrullus (watermelon) produce large amounts of waste and by-products, among them being discarded seeds. These by-products are an inexpensive raw material and a reliable source of bioactive phytochemicals, including antioxidant-rich polyphenols, tannins, flavonoids, and other components nutritionally important as essential fatty acids, dietary fiber, and minerals [8]. Cucurbitaceae seeds such as pumpkin, watermelon, and melon contain many nutrients such as protein, fibers, and minerals, highlighting their potential as a dietary supplement and a source of nutraceuticals; these potential applications contribute to the valorization of processing fruits by-products [9]. Pumpkin seed oil has been promoted as a new functional food and it is already produced and marketed as a healthy, edible cooking oil in some countries [10].
Sicana odorifera, or “Kurugua” is from the Cucurbitaceae family, found natively in the Latin American region, where it is widely used in folk medicine for various ailments; however, it is a species that has been hardly studied [4]. In the case of S. odorifera fruits, although it is not a fruit for mass consumption, it is precisely the lack of a market for its bio-waste that has limited its integral use, including the parts that represent the greatest loss, such as the peels (pericarp) and seeds (endocarp). Its crop is a strategy to increase food security and a family farming source [11]. The fruit itself has been used as a repellent, clothing perfume, or hot infusion with therapeutic uses in alternative medicine, inserted in popular wisdom at the Latin American level [12]. However, knowledge about the validated bioactive properties of these inedible parts (peel and seeds) is still limited. The black kurugua fruit (Sicana odorifera Naudim Vell.) is considered an exotic fruit; the large fruit contains 6% of its weight as seeds, in numbers from 900 to 1100 seeds per fruit. The epicarp of the fruit is intense purple, 30 to 60 cm in longitudinal diameter, and 9 to 15 cm in transverse diameter, and the pulp or mesocarp is approximately 2 cm thick, with oval seeds arranged in a row in the membranous endocarp. This bio-residue could have a great economic impact for the industrial processing of the pulp in the value chain, being, together with the peel, its main waste [11]. Studies on the use of its peel as a potential source of natural colorants have been published [13]. In S. odorifera seeds, insect repellent activity has been reported with aromatic properties. Triterpenes and flavonoids, including karounidiol dibenzoate, Cucurbita-5,23-diene-3h,25-diol, taxifolin, and quercetin were isolated from them [14]. The sweet aroma of the fruit, as well as the intense color of the peel, has been characterized [15,16,17][15][16][17]. The aromatic spectrum of the pulp is characteristic and the compounds responsible for the flavor were described (94.8% free volatile; with 61.1% as aliphatic alcohols [16], whereas studies on the composition of the seeds and the bioactivity of its components are still scarce. The phytochemical profile of the pulp is promising as a source of antioxidant compounds [18]. Regarding the peels, flavonols and anthocyanins with antioxidant activity have been described [17]. There are several animal models to evaluate the hepatoprotective effect of natural products; one of the effects is the model of liver damage induced by acetaminophen (APAP), a drug widely used as an antipyretic and analgesic, which in high doses can produce necrosis and insufficiency acute hepatica [19,20,21][19][20][21].
Liver disease is one of the leading causes of death in the world, and there is still an urgent demand for effective and safe hepatoprotective agents, despite advances in modern pharmacology [22]. The hepatoxicity of APAP is primarily caused by metabolism by cytochrome P450 to produce N-acetyl-p-benzoquinone imine (NAPQI), which can react with glutathione (GSH) to cause oxidative stress that can trigger the mitochondrial signal pathway and cause cell damage [23]. On the other hand, the solid evidence that demonstrates the multiple healthy effects of-3 PUFA for humans has stimulated the consumption of ω-3 PUFA. Its supply is limited, and is focused mainly on the consumption of fatty fish or bluefish and nutritional supplements based on fish oils or microalgae, thus hindering the increase in the consumption of these fatty acids in the western population. The incipient industrial production of vegetable oils rich in ALA in some Latin American countries is a novel and innovative alternative to increase the consumption and production of ω-3 fatty acids, specifically from its metabolic precursor, ALA [24,25][24][25]. Currently, plant residues in the form of S. odorifera seeds represent an opportunity to explore their properties and to give an integral use to industrialized fruits, following the current trend of generating more sustainable alternative processes, and the interest in the sustainable production of bioactive molecules [3]. The aim of this work was to describe the proximate composition, minerals and antioxidant activity of S. odorifera seeds and their fatty acids profile as well as the profile of polyphenol compounds, acute toxicity, behavior and hepatoprotective effect in mice of the methanolic extract, to explore their nutritional and nutraceutical potential in order to promote the use of this bio-residue.
2. Seeds Composition
2.1. Proximate and Minerals Composition
The results of the physical properties, proximate composition, minerals, and caloric value of the pulp and seeds of S. odorifera fruits are shown in Table 1. The mature fruits show an oblong shape, dark purple color in the epicarp (peel), and orange color in the mesocarp (pulp). The mesocarp had a more intense lightness color than that of the seeds. The seeds were bicolor, both brown and beige. The main components of the seeds were lipids and dietary fiber, whereas the mesocarp showed more water and total carbohydrate. Thus, their caloric value was higher than that of the pulp. On the mineral composition of the seeds, potassium, was predominant, followed by magnesium and calcium, while zinc and calcium were major in the pulp (Table 1).
Table 1. Sicana odorifera seeds and mesocarp physical characterization, proximate and minerals composition.
| Parameter | Mesocarp (Fresh Weight) |
Seeds (Dry Base) |
|---|---|---|
| Weight (g) | 1970 ± 51 | 0.11 ± 0.01 |
| Color | L* = 66.00 ± 2.45 a* = 11.71 ± 3.28 b* = 69.43 ± 2.32 |
L* = 34.80 ± 10.44 a* = 4.50 ± 5.52 b* = 8.70 ± 3.07 |
| Longitudinal diameter (cm) | 26.90 ± 1.4 | 1.58 ± 0.12 |
| Transverse diameter (cm) | 10.42 ± 0.7 | 0.80 ± 0.04 |
| Water (g/100 g) | 86.70 ± 0.4 | 10.06 ± 0.30 |
| Total lipids (g/100 g) | 1.31 ± 0.02 | 35.51 ± 0.40 |
| Ash (g/100 g) | 0.13 ± 0.01 | 2.55 ± 0.10 |
| Total protein (g/100 g) | 1.07 ± 0.08 | 18.05 ± 0.56 |
| Total carbohydrate (g/100 g) | 7.35 ± 0.31 | 2.80 ± 0.06 |
| Dietary fiber (g/100 g) | 3.11 ± 0.00 | 34.67 ± 0.31 |
| Caloric value (Kcal/100 g) | 45.5 ± 5 | 403 ± 5 |
| Fe (mg/100 g) | 0.35 ± 0.06 | 6.35 ± 0.69 |
| Mn (mg/100 g) | 0.55 ± 0.02 | 1.82 ± 0.10 |
| Cu (mg/100 g) | 0.25 ± 0.02 | 0.69 ± 0.09 |
| Zn (mg/100 g) | 42.31 ± 0.05 | 2.31 ± 0.09 |
| Mg (mg/100 g) | 5.16 ± 0.07 | 177.00 ± 4.66 |
| Ca (mg/100 g) | 29.61 ± 2.35 | 124.98 ± 9.17 |
| Na (mg/100 g) | 4.27 ± 0.54 | 29.51 ± 1.27 |
| K (mg/100 g) | Nd | 784.05 ± 52.40 |
The values are means ± DS (n = 10). Determinations made in fresh samples. Nd = no detected. * L: lightness, a* and b*; Coordinates that represent variation between reddish-greenish and yellowish-bluish, respectively.
2.2. Chemical Characterization through UHPLC-DAD and UHPLC-ESI-MS of the S. odorifera Seeds’ Extract
The MeOH extract of the Paraguayan kurugua (S. odorifera) seeds was analyzed employing UHPLC-DAD-ESI-MS/MS. The UHPLC-DAD profile at 254 and 350 nm are depicted in Figure 1, while the extracted ion chromatograms of each detected compound in negative and positive ion modes are shown in Figure 2 and Figure 3, respectively. The UHPLC-MS analysis allowed the tentative identification of 11 compounds in our samples, including one hydroxycinnamic acid derivative, one phenolic acid, four flavonols conjugates, three flavonol aglycones, and two cucurbitacins (Table 2).
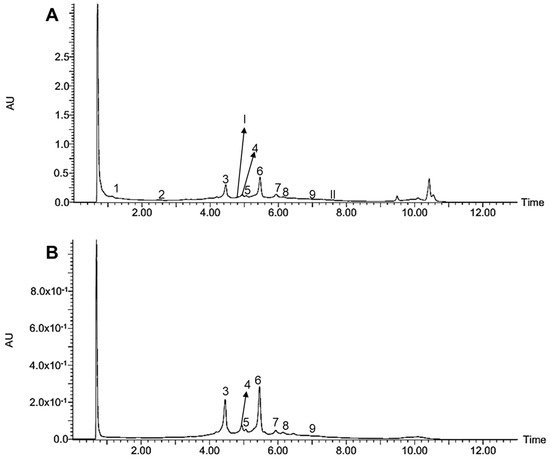
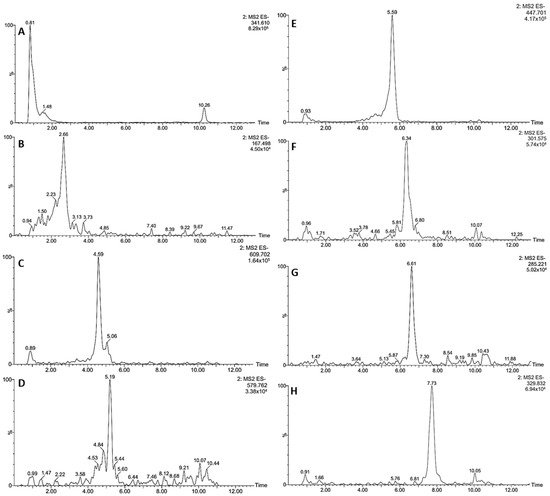

Figure 1. UHPLC-DAD profiles of the MeOH extract of S. odorifera seeds at 254 nm (A) and 350 nm (B).

Figure 2. Extracted ion chromatograms in negative ion mode of the compounds tentatively identified in S. odorifera seeds’ MeOH extract: Caffeoyl hexoside (A), Vanillic acid (B), Quercetin hexoside rhamnoside 1 and 2 (C), Quercetin pentoside rhamnoside (D), Quercetin rhamnoside (E), Quercetin (F), Luteolin (G), Quercetin dimethyl ether (H).

Figure 3. Extracted ion chromatograms in positive ion mode of the compounds tentatively identified in S. odorifera seeds’ MeOH extract: Boeticol (A), Karounidiol dibenzoate (B).
Table 2. Tentative identification of the compounds detected in the UHPLC-ESI-MS/MS profile of the MeOH seeds extract of S. odorifera.
| Peak | Rt (Min) | UVmax | [M − H]−/[M + H]+ | Polarity | MS/MS Fragments | Tentative Identification |
|---|---|---|---|---|---|---|
| 1 | 1.22–1.48 | 341.61 | Negative | 179.48 (45), 119.14 (100) | Caffeoyl hexoside | |
| 2 | 2.66 | 290, 265 | 167.49 | Negative | 108.22 (100) | Vanillic acid |
| 3 | 4.59 | 351, 265 | 609.91 | Negative | 608.75 (20), 300.68 (100), 179.27 (20) | Quercetin hexoside rhamnoside 1 |
| 4 | 5.06 | 350, 265 | 609.70 | Negative | 301.50 (100) | Quercetin hexoside rhamnoside 2 |
| 5 | 5.19 | 350 | 579.76 | Negative | 301.36 (100) | Quercetin pentoside rhamnoside |
| 6 | 5.59 | 346, 265 | 447.96 | Negative | 300.46 (100), 271.19 (20), 255.11 (20), 179.29 (25) | Quercetin rhamnoside |
| 7 | 6.34 | 301.58 | Negative | 150.92 (100), 107.37 (60) | Quercetin | |
| 8 | 6.61 | 285.22 | Negative | 175.08 (100), 151.22 (15) | Luteolin | |
| Positive | 350.169 (30), 262.64 (65), 232.61 (100) | Boeticol | ||||
| II | 7.84–8.01 | 647.25 | Positive | 647.16 (85), 226.95 (87), 171.56 (40), 104.39 (100) | Karounidiol dibenzoate |
* The compounds detected in positive ion mode are depicted in roman numerals (I and II).
The tentative assignation of the detected compounds was based on their retention time (Rt), UV absorption maxima, pseudomolecular ions, and MS/MS fragmentation patterns, compared to literature when available (Table 2).
The only hydroxycinnamic acid derivative was compound 1, which showed a pseudomolecular ion at m/z 341, losing a hexose (162 amu) to afford a secondary ion at m/z 179, suggesting a caffeoyl moiety [39][26]. Therefore, compound 1 was tentatively assigned as caffeoyl hexoside. Compound 2 was tentatively identified as vanillic acid, based on its (M − H)− ion at m/z 167 yielding and MS2 base peak at m/z 108; both characteristics of this phenolic acid. The UV absorption maxima around 260 and 290 nm supported the assignation [40][27].
Among flavonol conjugates, the peaks showed absorption maxima around 350 nm and an MS2 base peak at m/z 301; characteristics of a quercetin core [17]. Thus, compounds 3, 4, 5, and 6 were tentatively identified as quercetin derivatives (Table 1). The first two displayed a pseudomolecular ion at m/z 609, losing a hexosyl rhamnoside moiety (308 amu) to yield the base peak at m/z 301 in both cases. However, the retention time was different, suggesting isomeric structures. Therefore, compounds 3 and 4 were tentatively assigned as quercetin hexoside rhamnoside 1 and 2, respectively. Peak 5 showed an (M − H)− ion at m/z 579 and a neutral loss of a pentosyl rhamnoside moiety (278 amu) to afford the base peak at m/z 301. Thus, compound 5 was tentatively identified as quercetin pentoside rhamnoside [41][28].
The last (6) was tentatively assigned as quercetin rhamnoside because it exhibited a pseudomolecular ion at m/z 447 and a neutral loss of rhamnose (146 amu). The compounds 3 and 6 showed the most intense signals in the chromatographic profile (Figure 1), constituting the major compounds in the MeOH extract of S. odorifera seeds.
Further, three other signals were detected in the kurugua seeds, in agreement with flavonol aglycones. The first peak (7) exhibited a pseudomolecular ion at m/z 301 and MS/MS fragments at m/z 151 and 107, compatible with quercetin [42][29]. Comparatively, the second peak (8) showed an (M − H)− ion at m/z 285 and secondary ions at m/z 175 and 151, in agreement with luteolin.
The last peak (9) displayed an (M − H)− ion at m/z 329, losing a methyl (14 amu) to yield an intense fragment at m/z 314, suggesting the presence of quercetin dimethyl ether [43][30]. Therefore, compounds 7, 8, and 9 were tentatively assigned as quercetin, luteolin, and quercetin dimethyl ether, respectively.
The analysis in the positive ion mode allowed the detection of two cucurbitacins. Their extracted ion chromatograms are shown in Figure 3. The first (I) showed an [M + H]+ ion at m/z 443 and was tentatively assigned as boeticol, while compound II with a pseudomolecular ion of 647 amu was compatible with karounidiol dibenzoate. The assignation of compound I was supported by the intense fragments at m/z 263 and 233, in line with cucurbitane-type triterpenoids [44][31]. Finally, the identity of compound II was supported by the MS/MS fragments at m/z 227, 171, and 105, characteristics of Karounidiol dibenzoate [45][32].
2.3. Seeds Fatty Acids Profile by GC-MS
The results of the analysis of the fatty acid profile of the seeds have shown that the fatty acids are preferably polyunsaturated (Table 3).
Table 3. Sicana odorifera seeds’ fatty acids GC-Ms profile.
| Fatty Acids | Abbreviated Formula | mg/100 g |
|---|---|---|
| Miristic | C14:0 | 0.034 ± 0.02 |
| Pentadecanoic | C15:0 | 0.01 ± 0.01 |
| Palmitic | C16:0 | 3.64 ± 0.0 |
| Palmitoleic | C16:1 | 0.02 ± 0.01 |
| Margaric | C17:0 | 0.04 ± 0.01 |
| Stearic | C18:0 | 2.33 ± 0.02 |
| Oleic | ||
| Arachidic | ||
| C20:0 | 0.09 ± 0.01 | |
| Gondoic | C20:1 | 0.12 ± 0.01 |
| Total SFA | 18.09 ± 0.09 | |
| Total MUFA | 13.10 ± 0.06 | |
| Total PUFA | 68.68 ± 0.574 |
The values are expressed as mean ± SD (n = 3). SFA: Saturated fatty acids, MUFA: Monounsaturated fatty acids, PUFA: Polyunsaturated fatty acids.
3. Biological Assays
3.1. Antioxidant Activity
The antioxidant activity was measured as a function of the TPC concentration, monomeric anthocyanins, and vitamin C, in addition to the total antioxidant capacity by inhibition of the radical ABTS, the results of which are shown in Table 4.
Table 4. S. odorifera seed’s antioxidant potential.
| Parameter | Seeds (SBS) |
|||||
|---|---|---|---|---|---|---|
| TPC (mg GAE/100 g FW) | 47.34 ± 4.41 | |||||
| Monomeric anthocyanins (mg/100 g of cyanidin 3-glucoside) | 5.90 ± 0.98 | |||||
| Vitamin C (mg/100 g) | 1.11 ± 0.27 | |||||
| Total antioxidant capacity ABTS (μM TEAC/g) | 7.47 ± 0.62 | |||||
| C18:1c | ||||||
| 4.32 ± 0.02 | ||||||
| Linoleic | C18:2 ω6 | 9.98 ± 0.19 | ||||
| 9 | 7.73 | 329.83 | Negative | 314.78 (100) | Quercetin dimethyl ether | |
| 8,11 Octadecadienoic | C18:2 c | 0.40 ± 0.01 | I | 4.96 | ||
| Alfa Linolenic | C18:3 ω3 | 12.93 ± 0.01443.59 |
The values are means ± DS (n = 3). Determinations made in fresh samples. TPC: Total phenolic compounds.
3.2. Effects of S. odorifera Seeds’ Methanolic Extract on Acute Toxicity and the General Behavior Test on Mice
Oral administration (500, 1000, and 2000 mg/kg) of the methanolic extract of S. odorifera seeds did not cause lethality in female mice after 24 h of observation.
After 14 days of observation, the mice were euthanized, and no signs of morpho-anatomical alteration were observed in the internal organs macroscopically evaluated and compared with the organs corresponding to the blank group. The methanolic extract of S. odorifera seeds administered orally up to a dose of 2000 mg/kg was shown to be safe because there was no lethality or symptoms indicative of acute toxicity under the experimental working conditions.
No relevant effects on the general behavior were observed in mice of both sexes administered orally with different doses (10, 100, 300, and 500 mg/kg) of the extract. The most representative effects on the general behavior of the mice were observed within 4 h after administration and are recorded. The appearance of piloerection and self-cleaning behavior is observed in the group treated with the methanolic extract compared to the group treated with the blank. After this time, the influence on the parameters decreased until the total disappearance of these nonspecific effects, which were of a short duration and because they are the crude extract, are considered irrelevant.
3.3. Hepatoprotective Activity Assay of S. odorifera Seeds’ Methanolic Extract
We have studied the activity of S. odorifera seeds’ extract on acetaminophen-induced hepatotoxicity.
The influence of the oral administration of the seed extract on the serum levels of Albumin, total protein, and serum alkaline phosphatase of male mice with hepatic damage induced with acetaminophen is depicted in Figure 4. The oral administration of the methanolic extract of the seeds of S. odorifera did not show significant effects on the serum levels of alkaline phosphatase, total proteins, and albumin between the different treatments.
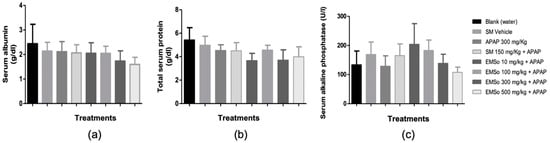

Figure 4. Influence of the oral administration of the seeds extract by group: (a) Variation of serum albumin levels of male mice in the different treatment groups; (b) Variation of serum levels of total proteins of male mice in the different treatment groups (c) Variation of serum alkaline phosphatase levels in male mice in the different treatment groups. Data are plotted as mean ± SD of six animals per group (n = 6). The statistical analysis used was one-way ANOVA followed by the Tukey test, where p < 0.05 was considered statistically significant.
In the present study, it was demonstrated that the administration of acetaminophen, silymarin, and the different doses (10, 100, 300, and 500 mg/kg) of the methanolic extract of S. odorifera seeds (EMSo) did not produce a significant change in serum albumin, serum total proteins, and serum alkaline phosphatase values. On the other hand, Figure 5 show the influence of the oral administration of the extract on the serum levels of glutamic-pyruvic transaminase and Figure 6 show glutamic-oxaloacetic transaminase of male mice with hepatic damage induced with acetaminophen.
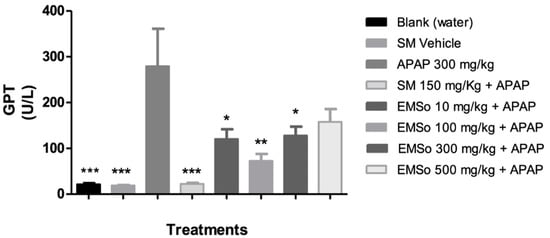
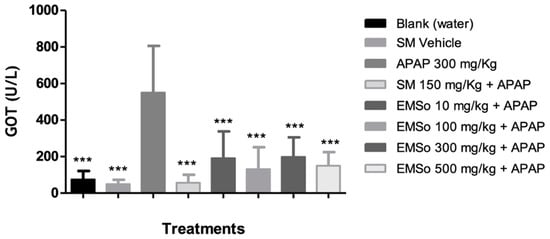

Figure 5. Influence of the oral administration of the seeds extract by group: Variation of serum levels of glutamic-pyruvic transaminase (GPT) of male mice in the different treatment groups. Data are plotted as mean ± SD of six animals per group (n = 6). The statistical analysis used was one-way ANOVA followed by the Tukey test, where p < 0.05 was considered statistically significant (* p < 0.05, ** p < 0.01, *** p < 0.001). SM; sylimarine, APAP; acetaminophen, EMSo; metanolic extract.

Figure 6. Influence of the oral administration of the seeds extract by group: variation of serum levels of glutamic-oxaloacetic transaminase (GOT) of male mice in the different treatment groups. Data are plotted as mean ± SD of six animals per group (n = 6). The statistical analysis used was one-way ANOVA followed by the Tukey test, where p < 0.05 was considered statistically significant (*** p < 0.001). SM; sylimarine, APAP; acetaminophen, EMSo; metanolic extract.
References
- FAO. Food Loss and Waste and the Right to Adequate Food: Making the Connection; FAO: Rome, Italy, 2018; ISBN 978-92-5-130932-2.
- Gómez-García, R.; Campos, D.A.; Aguilar, C.N.; Madureira, A.R.; Pintado, M. Biological Protein Precipitation: A Green Process for the Extraction of Cucumisin from Melon (Cucumis Melo L. Inodorus) by-Products. Food Hydrocoll. 2021, 116, 106650.
- Fierascu, R.C.; Sieniawska, E.; Ortan, A.; Fierascu, I.; Xiao, J. Fruits By-Products—A Source of Valuable Active Principles. A Short Review. Front. Bioeng. Biotechnol. 2020, 8, 319.
- Lima, J.F.; Silva, M.P.L.; Teles, S.; Silva, F.; Martins, G.N. Avaliação de diferentes substratos na qualidade fisiológica de sementes de melão de caroá . Rev. Bras. Plantas Med. 2010, 12, 163–167.
- Banerjee, J.; Singh, R.; Vijayaraghavan, R.; MacFarlane, D.; Patti, A.F.; Arora, A. Bioactives from Fruit Processing Wastes: Green Approaches to Valuable Chemicals. Food Chem. 2017, 225, 10–22.
- Bhat, I.U.H.; Bhat, R. Quercetin: A Bioactive Compound Imparting Cardiovascular and Neuroprotective Benefits: Scope for Exploring Fresh Produce, Their Wastes, and By-Products. Biology 2021, 10, 586.
- FAO. Fruit and Vegetables—Your Dietary Essentials: The International Year of Fruits and Vegetables, 2021, Background Paper; FAO: Rome, Italy, 2020; ISBN 978-92-5-133709-7.
- Nunes, P.; Bhat, R. Chapter 15—Valorization of Seeds of the Genera Cucumis, Citrullus, and Cucurbita. In Valorization of Agri-Food Wastes and By-Products; Bhat, R., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 317–329. ISBN 978-0-12-824044-1.
- Rezig, L.; Chouaibi, M.; Meddeb, W.; Msaada, K.; Hamdi, S. Chemical Composition and Bioactive Compounds of Cucurbitaceae Seeds: Potential Sources for New Trends of Plant Oils. Process Saf. Environ. Prot. 2019, 127, 73–81.
- Yao, Y.; Xu, B. New Insights into Chemical Compositions and Health Promoting Effects of Edible Oils from New Resources. Food Chem. 2021, 364, 130363.
- Imas González, B.A. Análisis Técnico y Económico de La Producción de Kurugua Sicana Odorifera (Vell.) Naud; Como Complemento En La Agricultura Familiar; Universidad Nacional de Asunción: San Lorenzo, Paraguay, 2009.
- De Filho, G.X.P.; Barreira, T.F.; Pinheiro, S.S.; de Cardoso, L.M.; Martino, H.S.D.; Pinheiro-Sant’Ana, H.M. ‘Melão Croá’ (Sicana Sphaerica Vell.) and ‘Maracujina’ (Sicana Odorifera Naud.): Chemical Composition, Carotenoids, Vitamins and Minerals in Native Fruits from the Brazilian Atlantic Forest. Fruits 2015, 70, 341–349.
- Mereles, L.; Caballero, S.; Burgos-edwards, A.; Benítez, M.; Ferreira, D.; Coronel, E. Extraction of Total Anthocyanins from Sicana Odorifera Black Peel Fruits Growing in Paraguay for Food Applications. Appl. Sci. 2021, 11, 6026.
- Nakano, S.; Fujimoto, Y.; Takaishi, Y.; Osorio, C.; Duque, C. Cucurbita-5,23-Diene-3β,25-Diol from Sicana Odorifera. Fitoterapia 2004, 75, 609–611.
- Kienteka, S.S.; Corrêa-Ferreira, M.L.; de Oliveira Petkowicz, C.L. Characterization of Cell Wall Polysaccharides from Sicana Odorifera Fruit and Structural Analysis of a Galactan-Rich Fraction Pectins as Side Chains. Carbohydr. Polym. 2018, 197, 395–402.
- Parada, F.; Duque, C.; Fujimoto, Y. Free and Bound Volatile Composition and Characterization of Some Glucoconjugates as Aroma Precursors in Melón de Olor Fruit Pulp (Sicana Odorifera). J. Agric. Food Chem. 2000, 48, 6200–6204.
- Jaramillo, K.; Dawid, C.; Hofmann, T.; Fujimoto, Y.; Osorio, C. Identification of Antioxidative Flavonols and Anthocyanins in Sicana Odorifera Fruit Peel. J. Agric. Food Chem. 2011, 59, 975–983.
- Albuquerque, B.R.; Dias, M.I.; Pereira, C.; Petrović, J.; Soković, M.; Calhelha, R.C.; Oliveira, M.B.P.P.; Ferreira, I.C.F.R.; Barros, L. Valorization of Sicanaodorifera (Vell.) Naudin Epicarp as a Source of Bioactive Compounds: Chemical Characterization and Evaluation of Its Bioactive Properties. Foods 2021, 10, 700.
- Abdel-Azeem, A.S.; Hegazy, A.M.; Ibrahim, K.S.; Farrag, A.-R.H.; El-Sayed, E.M. Hepatoprotective, Antioxidant, and Ameliorative Effects of Ginger (Zingiber Officinale Roscoe) and Vitamin E in Acetaminophen Treated Rats. J. Diet. Suppl. 2013, 10, 195–209.
- Shu, Y.; He, D.; Li, W.; Wang, M.; Zhao, S.; Liu, L.; Cao, Z.; Liu, R.; Huang, Y.; Li, H.; et al. Hepatoprotective Effect of Citrus Aurantium L. Against APAP-Induced Liver Injury by Regulating Liver Lipid Metabolism and Apoptosis. Int. J. Biol. Sci. 2020, 16, 752–765.
- Du, K.; Ramachandran, A.; Jaeschke, H. Oxidative Stress during Acetaminophen Hepatotoxicity: Sources, Pathophysiological Role and Therapeutic Potential. Redox Biol. 2016, 10, 148–156.
- Xu, G.-B.; Xiao, Y.-H.; Zhang, Q.-Y.; Zhou, M.; Liao, S.-G. Hepatoprotective Natural Triterpenoids. Eur. J. Med. Chem. 2018, 145, 691–716.
- Hinson, J.A.; Roberts, D.W.; James, L.P. Mechanisms of Acetaminophen-Induced Liver Necrosis. In Adverse Drug Reactions; Uetrecht, J., Ed.; Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2010; pp. 369–405. ISBN 978-3-642-00663-0.
- OPS/OMS. Plan de Acción Para Eliminar Los Ácidos Grasos Trans de Producción Industrial 2020–2025 En: 71a Sesión Del Comite Regional de La OMS Para Las Américas; OPS/OMS: Washington, DC, USA, 2019.
- Pipoyan, D.; Stepanyan, S.; Seda, S.; Romina, M.; Merendino, N.; Beglaryan, M.; Costantini, L. The Effect of Trans Fatty Acids on Human Health: Regulation and Consumption Patterns. Foods 2021, 10, 2452.
- Clifford, M.N.; Wu, W.; Kirkpatrick, J.; Kuhnert, N. Profiling the Chlorogenic Acids and Other Caffeic Acid Derivatives of Herbal Chrysanthemum by LC−MSn. J. Agric. Food Chem. 2007, 55, 929–936.
- Gruz, J.; Novák, O.; Strnad, M. Rapid Analysis of Phenolic Acids in Beverages by UPLC–MS/MS. Food Chem. 2008, 111, 789–794.
- Jaiswal, R.; Karaköse, H.; Rühmann, S.; Goldner, K.; Neumüller, M.; Treutter, D.; Kuhnert, N. Identification of Phenolic Compounds in Plum Fruits (Prunus Salicina L. and Prunus Domestica L.) by High-Performance Liquid Chromatography/Tandem Mass Spectrometry and Characterization of Varieties by Quantitative Phenolic Fingerprints. J. Agric. Food Chem. 2013, 61, 12020–12031.
- Schmidt, J. Negative Ion Electrospray High-Resolution Tandem Mass Spectrometry of Polyphenols. J. Mass Spectrom. 2016, 51, 33–43.
- Yasir, M.; Sultana, B.; Nigam, P.S.; Owusu-Apenten, R. Antioxidant and Genoprotective Activity of Selected Cucurbitaceae Seed Extracts and LC-ESIMS/MS Identification of Phenolic Components. Food Chem. 2016, 199, 307–313.
- Akihisa, T.; Yasukawa, K.; Kimura, Y.; Takido, M.; Kokke, W.C.M.C.; Tamura, T. 7-OX0-10α-Cucurbitadienol from the Seeds of Trichosanthes Kirilowii and Its Anti-Inflammatory Effect. Phytochemistry 1994, 36, 153–157.
- Ukiya, M.; Akihisa, T.; Tokuda, H.; Toriumi, M.; Mukainaka, T.; Banno, N.; Kimura, Y.; Hasegawa, J.; Nishino, H. Inhibitory Effects of Cucurbitane Glycosides and Other Triterpenoids from the Fruit of Momordica Grosvenori on Epstein−Barr Virus Early Antigen Induced by Tumor Promoter 12-O-Tetradecanoylphorbol-13-Acetate. J. Agric. Food Chem. 2002, 50, 6710–6715.
More
