You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 3 by Georgeta Bocheva and Version 4 by Catherine Yang.
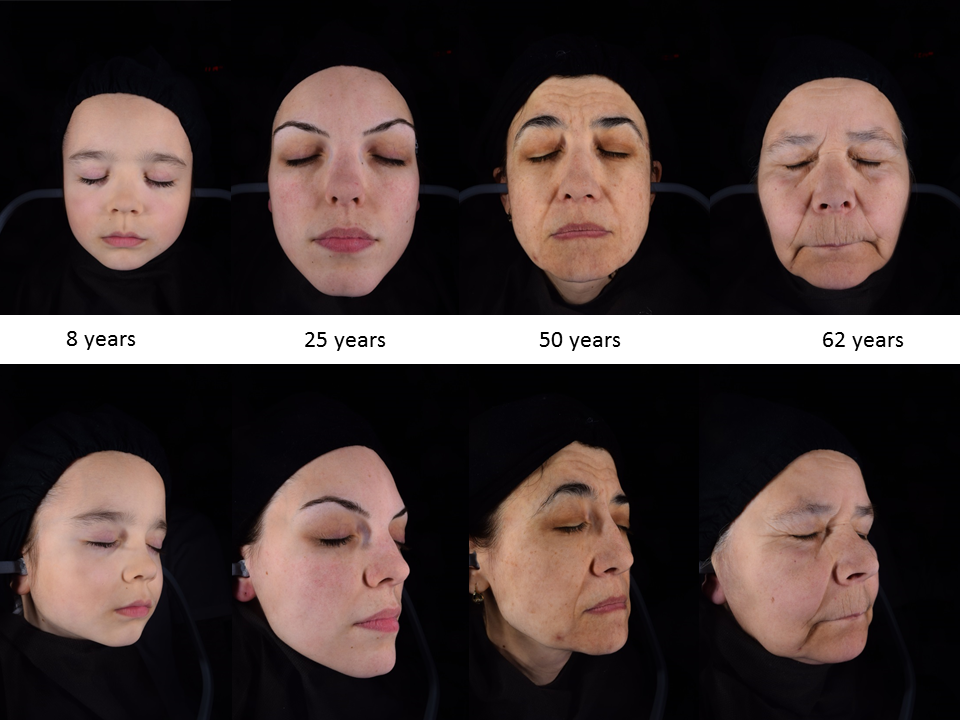
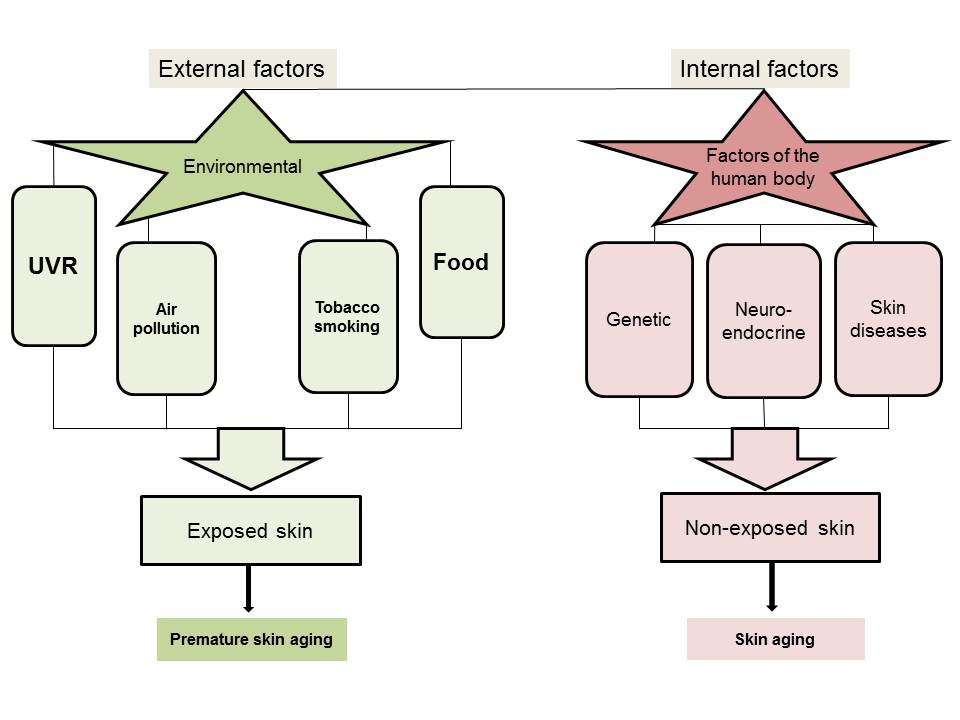
Skin aging is accompanied by a gradual loss of function, physiological integrity and the ability to cope with internal and external stressors. This is secondary to a combination of complex biological processes influenced by constitutive and environmental factors or by local and systemic pathologies. Skin aging and its phenotypic presentation are dependent on constitutive (genetic) and systemic factors. It can be accelerated by environmental stressors, such as ultraviolet radiation, pollutants and microbial insults.
- skin aging
- melatonin
- vitamin D
- photoprotection
 1. Introduction
1. Introduction
The skin is a complex multifunctional self-regulating organ in the human body. Its functions are critical to survival. The skin is not only a barrier that protects the organism from the deleterious insults of the external environment, but it is also crucial for thermoregulation, as well as its maintenance of electrolyte and fluid balance. Moreover, the skin also responds to environmental changes, such as biological, chemical, and physical factors, in order to regulate cutaneous and global body homeostasis [1][2][3].
It is well established that in the skin there is an important sophisticated network connecting cutaneous nerves and the local neuroendocrine and immune systems. The brain directly (via efferent nerves) or indirectly (via the adrenal glands or immune cells) regulates skin function. The neurocutaneous communication comprises of afferent and efferent nerves that release mediators acting on corresponding receptors expressed on skin cells [1][4]. Furthermore, as a sensory organ with neuroendocrine activities, the skin can also transmit humoral or neuronal signals to the central nervous, endocrine and immune systems. In addition, environmental factors or pathological processes induce skin changes that can imprint circulating immune cells acting as cellular messengers of skin responses to the changes in local homeostasis [1][2]. The skin also operates as a biofactory for the synthesis, processing and metabolism of the wide range of structural proteins, glycans, lipids and signaling molecules [5], as well as a fully functional neuroendocrine organ [6][7]. The human skin produces a variety of hormones, neuropeptides and neurotransmitters [1][2][3][8] in addition to the formation of vitamin D3 [9][10][11]. The skin responds to stress (such as UV light) by local synthesis of all hormones of the classical hypothalamic-pituitary-adrenal (HPA) axis [12]. Specifically, skin cells are capable of producing corticotropin-releasing hormone (CRH) [13][14][15][16][17][18][19][20][21], CRH-related peptides including urocortin 1 and 2 [3][22], proopiomelanocortin (POMC)-derived ACTH, α-MSH and β-endorphin [3][13][23][24][25][26][27][28], and glucocorticoids [29][30]. They also express the corresponding receptors. There are also many other hormones synthetized or activated/inactivated in the skin, including thyroid releasing hormone (TRH), thyroid stimulating hormone (TSH) and thyroid hormones, [31][32][33][34]; sex hormones and their precursors, as well as ∆7 steroids and different secosteroidal products [7][29][35][36][37][38]. The skin expresses the enzyme cytochrome P450scc (CYP11A1), which initiates steroid synthesis by converting cholesterol to pregnenolone in a similar manner as in other steroidogenic tissues [36][38][39][40][41][42][43][44][45]. In addition, skin cells can produce catecholamines [46][47], serotonin [48][49][50][51], and melatonin [48][50][52][53][54][55]. Indeed, melatonin and its biologically active metabolites are essential for physiological skin functions and protection against environmental stress [48][54][55][56][57][58].
2. Skin Aging
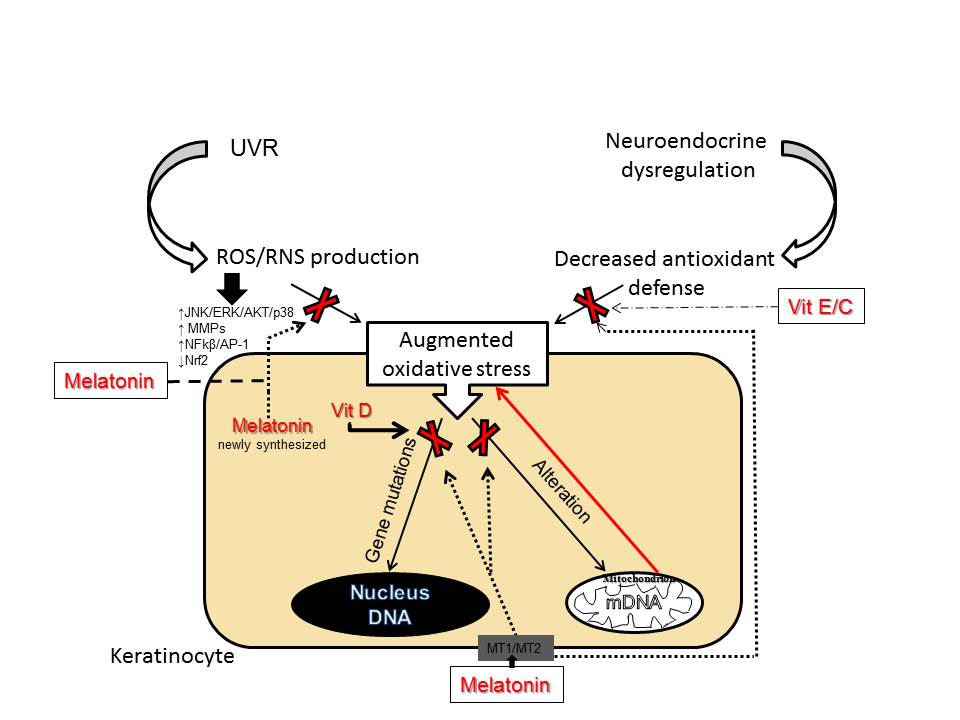
Aging is a natural process leading to the accumulation of damage and progressive deterioration in the biochemical, physiological and morphological functions on the systemic or organ levels [59][60]. Chronobiological aging mainly results from imbalanced endocrine circadian rhythmicity, which is linked to numerous health complications and pathologies in aging populations. Many factors can cause or aggravate hormone deficiencies (like nutritional, dietary, lifestyle, behavioral, environmental deficiencies, etc.) [61][62]. These hormonal changes induce morphological and functional alterations of all organs and systems, including the central nervous system (CNS )and skin. Moreover, the physiological aging process results in most of the phenotypic changes observed in the skin. There are age-related changes affecting all endocrine glands, which sometimes are so intertwined that the reduced function in one gland affects the other one [2][7][63]. Aging affects the expression of POMC and production of POMC-derived peptides, especially of melanocortin receptor 1 (MC1R) and MC2R agonists, which are of crucial importance for skin biological systems [2][64]. The regulation of the skin steroidogenic system cannot be underestimated, since it can regulate epidermal functions and skin immunity [7][38]. The breakdown of this steroidogenic activity can lead to pathological skin changes and diseases. The abnormal synthesis of skin cholesterol, involving a drastic reduction in steroids, is associated with down-regulation of epidermal differentiation [7][38][65]. Furthermore, the levels of steroidogenic acute regulatory protein (StAR) mRNA were found to gradually decrease in the skin tissues of elderly people, in contrast to younger ones [66]. With increasing age, the capacity of the skin to produce vitamin D3 declines, thus its protective effects are reduced [67][68]. Several factors contribute to this vitamin D deficiency state, such as behavior factors (limited sun exposure, malnutrition, etc.) and reduced synthetic capacity [69].
3. Anti-Aging Strategies
While aging as a natural phenomenon is genetically determined, premature photoaging can be prevented. Wrinkling and pigmentation are directly associated with premature skin aging and are considered to be the most critical skin events [70]. Photoprotection achieved by physical and chemical UV filters is the main preventive measure against skin photo-damage. Use of nutraceuticals (the term is derived from “nutrition” and “pharmaceutical” [71]) represent a promising strategy for preventing, delaying or minimizing the premature skin aging and age-associated diseases, including skin cancers [72]. Among them are plant polyphenols, bioactive peptides and oligosaccharides, carotenoids, vitamins and polyunsaturated fatty acids. Although some studies have reported that polyphenols can exert cytotoxic effect, polyphenolic compounds (curcumin; polyphenols from green tee, grape, soybeans, pomegranate, etc.) belong to the most frequently used ingredients in modern cosmeceutical and dermatological products [73][74][75][76][77]. Numerous studies suggest that polyphenols modulate the cellular inflammatory response of the NF-κβ pathway [78][79] and exert indirect antioxidant actions via activation of the Nrf2 [80]. Topical nicotinamide (niacinamide, vitamin B3) improves skin appearance and provides beneficial effects in prevention of the loss of dermal collagen that characterizes photoaging [81][82][83]. Vitamin B3, a precursor of Nicotinamide Adenine Dinucleotide (NAD), can also prevent UV-induced depletion of ATP in keratinocytes, leading to the acceleration of energy-dependent DNA repair processes [84]. When DNA damage cannot be repaired, an activation of poly-ADP-ribose-polymerase (PARP-1) induces apoptosis by activation NF-κβ pathway [85]. Hence, the UV-protective effects of vitamin B3 on the skin include regulation of cellular metabolism [86][87]. The ability of nicotinamide to enhance PARP-1 and regulate DNA repair mechanisms lead to its inclusion in regular sunscreens [88][89]. The potent antioxidant properties of vitamins C and E are well known and documented. They are widely used for skin care and in photo-protection, either as nutraceuticals or for topical application [90]. The incorporation of ferulic acid improves chemical stability of the vitamins (C + E) and increases photo-protection of photo-exposed skin [91][92][93]. Another preventive measure against premature skin aging is the usage of vitamin D3 derivatives. It was reported that active forms of vitamin D3 protect, attenuate, or even reverse UVB-induced cell and DNA damage in skin cells [67][94][95][96][97][98][99][100]. Unfortunately, the chronic use of vitamin D3 at therapeutic doses in its classical active forms including 1,25(OH)2D3 is severely limited due to its calcemic (toxic) effects. However, the discovery of an alternative pathway of vitamin D activation initiated by CYP11A1 [36][37][38], which produces biologically active but non-calcemic novel derivatives detectable in vivo [101][102][103][104], offers promises for therapeutic applications against photoaging and UVR induced skin pathology [105]. Vitamin D analogs may increase the DNA repair capacity in keratinocytes and melanocytes by enhancement of the expression of tumor suppressor protein p53 phosphorylated at Ser-15, but not at Ser-46 [106]. Phosphorylation at Ser-15 and Ser-20 of p53 activates p53 and promotes DNA repair, with phosphorylation of p53 at Ser-46 being responsible for regulation of apoptosis after DNA damage [107]. In addition, novel vitamin D derivatives produced by CYP11A1 down-regulate the formation of mutagenic and genotoxic cyclobutane pyrimidine dimers (CPD) produced after UVB exposure. Thus, both classical 1,25(OH)2D3 [95][96] and novel CYP11A1-derived 20(OH)D3 and 20,23(OH)2D3, and other vitamin D3 derivatives, may work as protectors of the human epidermis against UV-induced oxidative damage, not only in keratinocytes but also in melanocytes [106]. Vitamin D3, production of which in the skin is induced by solar radiation, is essentially important as a protector of skin homeostasis [108]. It can attenuate DNA- and metabolic-damage by reducing H2O2 and NO levels, elevating glutathione levels, and enhancing DNA repair. In advanced age, the capacity of the skin to produce vitamin D, which could be a part of this intrinsic protective mechanism against UV-damage, declines. Therefore, the supplementation of vitamin D is of great importance in the elderly population. The most promising candidate for delaying skin aging and for the treatment of several dermatoses associated with oxidative damage is melatonin. Melatonin is the main secretory hormonal product of the pineal gland and a regulator of chronobiological activities. Melatonin is also synthesized in numerous extrapineal sites including skin and hair follicles [54][58][109][110] where it can act on functional melatonin type 1 and 2 receptors (MT1 and MT2) [48][53][111][112][113][114][115]. Surprisingly, it was found that skin produces a much higher amount of melatonin for its own use than can be detected in serum [54][110]. Skin melatonin exerts multifaceted functions [114][115]. In addition to receptor-mediated actions, melatonin and its metabolites act as relevant direct antioxidants. Moreover, melatonin is one of the most potent free radical scavengers [116][117][118], even stronger than vitamins C and E [119]. Several in vitro studies have confirmed that melatonin and its metabolites can protect keratinocytes and melanocytes from UVB-induced damages. The mechanism of this protection includes activation of Nrf2 and upregulation of the Nrf2-related pathway [120][121]. Similarly, melatonin protects dermal fibroblasts from solar irradiation by increasing HO-1 expression and restoring the physiological expression of ECM proteins [122][123]. Melatonin reduces oxidative stress, not only as a direct ROS/RNS scavenger, but also indirectly via stimulation of antioxidant enzymes and inhibition of pro-oxidant enzymes [118][124]. Indeed, melatonin can upregulate expression of antioxidant genes [55][120][121][125]. Melatonin and its metabolites could also protect DNA from oxidative damages and reduce the levels of CPD’s or pyrimidine photoproducts (6-4PP) [120][126][127]. Melatonin, as an endogenous regulator, similarly to vitamin D3, stimulates phosphorylation of p53 at Ser-15 and enhances nucleotide excision repair (NER), thus preventing accumulation of damaged DNA and promoting antitumor activity [112][121][128]. Apart from its anti-oxidative properties, melatonin also preserves mitochondrial function. As we previously proposed, photoprotective functions of melatonin and its metabolites are directly or indirectly dependent on mitochondria, which appear to be a central hub of melatonin metabolism in skin cells [56]. Melatonin protects mitochondria not only directly, by ROS scavenging but also via maintenance of mitochondrial membrane potential and mitochondrial homeostasis in UV-exposed keratinocytes [56][129]. Additionally, melatonin and its metabolites ameliorate UVR-induced mitochondrial oxidative stress in human MNT-1 melanoma cells [130]. These data support the development of novel mitochondria-targeted antioxidants based on melatonin. Furthermore, the lightening effects of melatonin and some of its metabolites are due to inhibition of proliferation and tyrosinase activity in epidermal melanocytes [110]. Since melatonin and its metabolites over the years have proved their cytoprotective and antiaging properties, topical application of exogenous melatonin and/or metabolites would be a useful strategy against skin aging [131][132]. To enhance the protective effects and prevent wrinkle formation during photoaging, sunscreens and antioxidants (topical and systemic including vitamin C) often are combined with retinoids. The use of retinoids can promote collagen production [70]. Retinoids, especially retinoic acids (RAs) enhance the steroidogenic potential in many classical and non-classical steroidogenic tissues, which decrease due to hormonal imbalance in aging [7][29][133]. Local regulation of steroidogenic activity in keratinocytes of the epidermis is important for skin physiology and homeostasis. RAs improve wrinkled appearance, post-inflammatory hyperpigmentation and inhibit differentiation of keratinocytes in both mice and humans [30], but they often lead to irritation.References
- Slominski, A.; Wortsman, J. Neuroendocrinology of the skin. Endocr. Rev. 2000, 21, 457–487.
- Slominski, A.T.; Zmijewski, M.A.; Skobowiat, C.; Zbytek, B.; Slominski, R.M.; Steketee, J.D. Sensing the environment: Regulation of local and global homeostasis by the skin’s neuroendocrine system. Adv. Anat. Embryol. Cell Biol. 2012, 212, 1–115.
- Slominski, A.T.; Zmijewski, M.A.; Zbytek, B.; Tobin, D.J.; Theoharides, T.C.; Rivier, J. Key role of CRF in the skin stress response system. Endocr. Rev. 2013, 34, 827–884.
- Roosterman, D.; Goerge, T.; Schneider, S.W.; Bunnett, N.W.; Steinhoff, M. Neuronal control of skin function: The skin as a neuroimmunoendocrine organ. Physiol. Rev. 2006, 86, 1309–1379.
- Chuong, C.M.; Nickoloff, B.J.; Elias, P.M.; Goldsmith, L.A.; Macher, E.; Maderson, P.A.; Sundberg, J.P.; Tagami, H.; Plonka, P.M.; Thestrup-Pederson, K.; et al. What is the “true” function of skin? Exp. Dermatol. 2002, 11, 159–187.
- Zouboulis, C.C. Human skin: An independent peripheral endocrine organ. Horm. Res. 2000, 54, 230–242.
- Slominski, A.; Zbytek, B.; Nikolakis, G.; Manna, P.R.; Skobowiat, C.; Zmijewski, M.; Li, W.; Janjetovic, Z.; Postlethwaite, A.; Zouboulis, C.C.; et al. Steroidogenesis in the skin: Implications for local immune functions. J. Steroid. Biochem. Mol. Biol. 2013, 137, 107–123.
- Steinhoff, M.; Bienenstock, J.; Schmelz, M.; Maurer, M.; Wei, E.; Bíró, T. Neurophysiological, neuroimmunological, and neuroendocrine basis of pruritus. J. Invest. Dermatol. 2006, 126, 1705–1718.
- Bikle, D.D. Vitamin D: An ancient hormone. Exp. Dermatol. 2011, 20, 7–13.
- Lehmann, B.; Sauter, W.; Knuschke, P.; Dressler, S.; Meurer, M. Demonstration of UVB-induced synthesis of 1 alpha,25-dihydroxyvitamin D3 (calcitriol) in human skin by microdialysis. Arch. Dermatol. Res. 2003, 295, 24–28.
- Holick, M.F. Vitamin D: A millenium perspective. J. Cell. Biochem. 2003, 88, 296–307.
- Slominski, A.; Wortsman, J.; Tuckey, R.C.; Paus, R. Differential expression of HPA axis homolog in the skin. Mol. Cell. Endocrinol. 2007, 265-266, 143–149.
- Slominski, A.; Ermak, G.; Hwang, J.; Chakraborty, A.; Mazurkiewicz, J.E.; Mihm, M. Proopiomelanocortin, corticotropin releasing hormone and corticotropin releasing hormone receptor genes are expressed in human skin. FEBS Lett. 1995, 374, 113–116.
- Slominski, A.; Wortsman, J.; Pisarchik, A.; Zbytek, B.; Linton, E.A.; Mazurkiewicz, J.E.; Wei, E.T. Cutaneous expression of corticotropin-releasing hormone (CRH), urocortin, and CRH receptors. FASEB J. 2001, 15, 1678–1693.
- Zouboulis, C.C.; Seltmann, H.; Hiroi, N.; Chen, W.; Young, M.; Oeff, M.; Scherbaum, W.A.; Orfanos, C.E.; McCann, S.M.; Bornstein, S.R. Corticotropin-releasing hormone: An autocrine hormone that promotes lipogenesis in human sebocytes. Proc. Natl. Acad. Sci. USA 2002, 99, 7148–7153.
- Slominski, A.; Baker, J.; Ermak, G.; Chakraborty, A.; Pawelek, J. Ultraviolet B stimulates production of corticotropin releasing factor (CRF) by human melanocytes. FEBS Lett. 1996, 399, 175–176.
- Slominski, A.; Ermak, G.; Mazurkiewicz, J.E.; Baker, J.; Wortsman, J. Characterization of corticotropin-releasing hormone (CRH) in human skin. J. Clin. Endocrinol. Metab. 1998, 83, 1020–1024.
- Slominski, A.; Szczesniewski, A.; Wortsman, J. Liquid chromatography-mass spectrometry detection of corticotropin-releasing hormone and proopiomelanocortin-derived peptides in human skin. J. Clin. Endocrinol. Metab. 2000, 85, 3582–3588.
- Ito, N.; Ito, T.; Betterman, A.; Paus, R. The human hair bulb is a source and target of CRH. J. Invest. Dermatol. 2004, 122, 235–237.
- Zbytek, B.; Wortsman, J.; Slominski, A. Characterization of a ultraviolet B-induced corticotropin-releasing hormone proopiomelanocortin system in human melanocytes. Mol. Endocrinol. 2006, 20, 2539–2547.
- Slominski, A.T.; Botchkarev, V.; Choudhry, M.; Fazal, N.; Fechner, K.; Furkert, J.; Krause, E.; Roloff, B.; Sayeed, M.; Wei, E.; et al. Cutaneous expression of CRH and CRH-R. Is there a “skin stress response system?”. Ann. N. Y. Acad. Sci. 1999, 885, 287–311.
- Slominski, A.; Roloff, B.; Curry, J.; Dahiya, M.; Szczesniewski, A.; Wortsman, J. The skin produces urocortin. J. Clin. Endocrinol. Metab. 2000, 85, 815–823.
- Slominski, A.; Wortsman, J.; Luger, T.; Paus, R.; Solomon, S. Corticotropin releasing hormone and proopiomelanocortin involvement in the cutaneous response to stress. Physiol. Rev. 2000, 80, 979–1020.
- Kono, M.; Nagata, H.; Umemura, S.; Kawana, S.; Osamura, R.Y. In situ expression of corticotropin-releasing hormone (CRH) and proopiomelanocortin (POMC) genes in human skin. FASEB J. 2001, 15, 2297–2299.
- Skobowiat, C.; Dowdy, J.C.; Sayre, R.M.; Tuckey, R.C.; Slominski, A. Cutaneous hypothalamic-pituitary-adrenal axis homolog: Regulation by ultraviolet radiation. Am. J. Physiol. Endocrinol. Metab. 2011, 301, 484–493.
- Slominski, A.; Paus, R.; Mazurkiewicz, J. Proopiomelanocortin expression in the skin during induced hair growth in mice. Experientia 1992, 48, 50–54.
- Luger, T.A.; Paus, R.; Slominski, A.; Lipton, J. The proopiomelanocortin system in cutaneous neuroimmunomodulation. An introductory overview. Ann. N. Y. Acad. Sci. 1999, 885, xi.
- Slominski, A.; Wortsman, J.; Mazurkiewicz, J.E.; Matsuoka, L.; Dietrich, J.; Lawrence, K.; Gorbani, A.; Paus, R. Detection of proopiomelanocortin-derived antigens in normal and pathologic human skin. J. Lab. Clin. Med. 1993, 122, 658–666.
- Slominski, A.T.; Manna, P.R.; Tuckey, R.C. Cutaneous glucocorticosteroidogenesis: Securing local homeostasis and the skin integrity. Exp. Dermatol. 2014, 23, 369–374.
- Slominski, A.T.; Manna, P.R.; Tuckey, R.C. On the role of skin in the regulation of local and systemic steroidogenic activities. Steroids 2015, 103, 72–88.
- Slominski, A.; Wortsman, J.; Kohn, L.; Ain, K.B.; Venkataraman, G.M.; Pisarchik, A.; Chung, J.H.; Giuliani, C.; Thornton, M.; Slugocki, G.; et al. Expression of hypothalamic-pituitary-thyroid axis related genes in the human skin. J. Invest. Dermatol. 2002, 119, 1449–1455.
- Bodó, E.; Kany, B.; Gáspárm, E.; Knüver, J.; Kromminga, A.; Ramot, Y.; Bíróm, T.; Tiedem, S.; van Beek, N.; Poeggeler, B.; et al. Thyroid-stimulating hormone, a novel, locally produced modulator of human epidermal functions, is regulated by thyrotropin-releasing hormone and thyroid hormones. Endocrinology 2010, 151, 1633–1642.
- Gáspár, E.; Hardenbicker, C.; Bodó, E.; Wenzel, B.; Ramot, Y.; Funk, W.; Kromminga, A.; Paus, R. Thyrotropin releasing hormone (TRH): A new player in human hair-growth control. FASEB J. 2010, 24, 393–403.
- Paus, R. Exploring the “thyroid-skin connection”: Concepts, questions, and clinical relevance. J. Invest. Dermatol. 2010, 130, 7–10.
- Slominski, A.T.; Zmijewski, M.A.; Semak, I.; Zbytek, B.; Pisarchik, A.; Li, W.; Zjawiony, J.; Tuckey, R.C. Cytochromes p450 and skin cancer: Role of local endocrine pathways. Anticancer. Agents Med. Chem. 2014, 14, 77–96.
- Slominski, A.; Kim, T.K.; Zmijewski, M.A.; Janjetovic, Z.; Li, W.; Chen, J.; Kusniatsova, E.I.; Semak, I.; Postlethwaite, A.; Miller, D.D.; et al. Novel vitamin D photoproducts and their precursors in the skin. Dermatoendocrinol. 2013, 5, 7–19.
- Slominski, A.T.; Kim, T.K.; Li, W.; Yi, A.K.; Postlethwaite, A.; Tuckey, R.C. The role of CYP11A1 in the production of vitamin D metabolites and their role in the regulation of epidermal functions. J. Steroid Biochem. Mol. Biol. 2014, 144, 28–39.
- Slominski, A.T.; Li, W.; Kim, T.K.; Semak, I.; Wang, J.; Zjawiony, J.K.; Tuckey, R.C. Novel activities of CYP11A1 and their potential physiological significance. J. Steroid Biochem. Mol. Biol. 2015, 151, 25–37.
- Slominski, A.; Ermak, G.; Mihm, M. ACTH receptor, CYP11A1, CYP17 and CYP21A2 genes are expressed in skin. J. Clin. Endocrinol. Metab. 1996, 81, 2746–2749.
- Slominski, A.; Zjawiony, J.; Wortsman, J.; Semak, I.; Stewart, J.; Pisarchik, A.; Sweatman, T.; Marcos, J.; Dunbar, C.; Tuckey, R. A novel pathway for sequential transformation of 7-dehydrocholesterol and expression of the P450scc system in mammalian skin. Eur. J. Biochem. 2004, 271, 4178–4188.
- Thiboutot, D.; Jabara, S.; McAllister, J.M.; Sivarajah, A.; Gilliland, K.; Cong, Z.; Clawson, G. Human skin is a steroidogenic tissue: Steroidogenic enzymes and cofactors are expressed in epidermis, normal sebocytes, and an immortalized sebocyte cell line (SEB-1). J. Invest. Dermatol. 2003, 120, 905–914.
- Inoue, T.; Miki, Y.; Abe, K.; Hatori, M.; Hosaka, M.; Kariya, Y.; Kakuo, S.; Fujimura, T.; Hachiya, A.; Honma, S.; et al. Sex steroid synthesis in human skin in situ: The roles of aromatase and steroidogenic acute regulatory protein in the homeostasis of human skin. Mol. Cell. Endocrinol. 2012, 362, 19–28.
- Li, J.; Daly, E.; Campioli, E.; Wabitsch, M.; Papadopoulos, V. De novo synthesis of steroids and oxysterols in adipocytes. J. Biol. Chem. 2014, 289, 747–764.
- Tuckey, R.C. Progesterone synthesis by the human placenta. Placenta 2005, 26, 273–281.
- Miller, W.L.; Auchus, R.J. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr. Rev. 2011, 32, 81–151.
- Grando, S.A.; Pittelkow, M.R.; Schallreuter, K.U. Adrenergic and cholinergic control in the biology of epidermis: Physiological and clinical significance. J. Invest. Dermatol. 2006, 126, 1948–1965.
- Schallreuter, K.U.; Pittelkow, M.R.; Swanson, N.N.; Beazley, W.D.; Körner, C.; Ehrke, C.; Büttner, G. Altered catecholamine synthesis and degradation in the epidermis of patients with atopic eczema. Arch. Dermatol. Res. 1997, 289, 663–666.
- Slominski, A.; Wortsman, J.; Tobin, D.J. The cutaneous serotoninergic/melatoninergic system: Securing a place under the sun. FASEB J. 2005, 19, 176–194.
- Slominski, A.; Pisarchik, A.; Semak, I.; Sweatman, T.; Wortsman, J.; Szczesniewski, A.; Slugocki, G.; McNulty, J.; Kauser, S.; Tobin, D.J.; et al. Serotoninergic and melatoninergic systems are fully expressed in human skin. FASEB J. 2002, 16, 896–898.
- Slominski, A.; Semak, I.; Pisarchik, A.; Sweatman, T.; Szczesniewski, A.; Wortsman, J. Conversion of L-tryptophan to serotonin and melatonin in human melanoma cells. FEBS Lett. 2002, 511, 102–106.
- Slominski, A.; Pisarchik, A.; Semak, I.; Sweatman, T.; Szczesniewski, A.; Wortsman, J. Serotoninergic system in hamster skin. J. Invest. Dermatol. 2002, 119, 934–942.
- Slominski, A.; Baker, J.; Rosano, T.G.; Guisti, L.W.; Ermak, G.; Grande, M.; Gaudet, S.J. Metabolism of serotonin to N-acetylserotonin, melatonin, and 5-methoxytryptamine in hamster skin culture. J. Biol. Chem. 1996, 271, 12281–12286.
- Kobayashi, H.; Kromminga, A.; Dunlop, T.W.; Tychsen, B.; Conrad, F.; Suzuki, N.; Memezawa, A.; Bettermann, A.; Aiba, S.; Carlberg, C.; et al. A role of melatonin in neuroectodermal-mesodermal interactions: The hair follicle synthesizes melatonin and expresses functional melatonin receptors. FASEB J. 2005, 19, 1710–1712.
- Slominski, A.; Tobin, D.J.; Zmijewski, M.A.; Wortsman, J.; Paus, R. Melatonin in the skin: Synthesis, metabolism and functions. Trends Endocrinol. Metab. 2008, 19, 17–24.
- Slominski, A.T.; Kleszczyński, K.; Semak, I.; Janjetovic, Z.; Zmijewski, M.A.; Kim, T.K.; Slominski, R.M.; Reiter, R.J.; Fischer, T.W. Local melatoninergic system as the protector of skin integrity. Int. J. Mol. Sci. 2014, 15, 17705–17732.
- Slominski, A.T.; Zmijewski, M.A.; Semak, I.; Kim, T.K.; Janjetovic, Z.; Slominski, R.M.; Zmijewski, J.W. Melatonin, mitochondria, and the skin. Cell. Mol. Life Sci. 2017, 74, 3913–3925.
- Fischer, T.W.; Slominski, A.; Zmijewski, M.A.; Reiter, R.J.; Paus, R. Melatonin as a major skin protectant: From free radical scavenging to DNA damage repair. Exp. Dermatol. 2008, 17, 713–730.
- Fischer, T.W.; Slominski, A.; Tobin, D.J.; Paus, R. Melatonin and the hair follicle. J. Pineal Res. 2008, 44, 1–15.
- Harman, D. Aging: Overview. Ann. N. Y. Acad. Sci. 2001, 928, 1–21.
- Kirkwood, T.B.; Austad, S.N. Why do we age? Nature 2000, 408, 233–238.
- Clegg, A.; Young, J.; Iliffe, S.; Rikkert, M.O.; Rockwood, K. Frailty in elderly people. Lancet 2013, 381, 752–762.
- Hertoghe, T. The “multiple hormone deficiency” theory of aging: Is human senescence caused mainly by multiple hormone deficiencies? Ann. N. Y. Acad. Sci. 2005, 1057, 448–465.
- Makrantonaki, E.; Schönknecht, P.; Hossini, A.M.; Kaiser, E.; Katsouli, M.M.; Adjaye, J.; Schröder, J.; Zouboulis, C.C. Skin and brain age together: The role of hormones in the ageing process. Exp. Gerontol. 2010, 45, 801–813.
- Pain, S.; Dezutter, C.; Reymermier, C.; Vogelgesang, B.; Delay, E.; André, V. Age-related changes in pro-opiomelanocortin (POMC) and related receptors in human epidermis. Int. J. Cosmet. Sci. 2010, 32, 266–275.
- Cirillo, N.; Prime, S.S. Keratinocytes synthesize and activate cortisol. J. Cell. Biochem. 2011, 112, 1499–1505.
- Törmä, H. Regulation of keratin expression by retinoids. Dermatoendocrinol. 2011, 3, 136–140.
- De Haes, P.; Garmyn, M.; Verstuyf, A.; De Clercq, P.; Vandewalle, M.; Degreef, H.; Vantieghem, K.; Bouillon, R.; Segaert, S. 1,25-Dihydroxyvitamin D3 and analogues protect primary human keratinocytes against UVB-induced DNA damage. J. Photochem. Photobiol. B 2005, 78, 141–148.
- Slominski, A.T.; Brożyna, A.A.; Skobowiat, C.; Zmijewski, M.A.; Kim, T.K.; Janjetovic, Z.; Oak, A.S.; Jozwicki, W.; Jetten, A.M.; Mason, R.S.; et al. On the role of classical and novel forms of vitamin D in melanoma progression and management. J. Steroid. Biochem. Mol. Biol. 2018, 177, 159–170.
- MacLaughlin, J.; Holick, M.F. Aging decreases the capacity of human skin to produce vitamin D3. J. Clin. Invest. 1985, 76, 1536–1538.
- Ganceviciene, R.; Liakou, A.I.; Theodoridis, A.; Makrantonaki, E.; Zouboulis, C.C. Skin anti-aging strategies. Dermatoendocrinology 2012, 4, 308–319.
- Kalra, E.K. Nutraceutical—definition and introduction. AAPS PharmSci. 2003, 5, 25.
- Pérez-Sánchez, A.; Barrajón-Catalán, E.; Herranz-López, M.; Micol, V. Nutraceuticals for Skin Care: A Comprehensive Review of Human Clinical Studies. Nutrients 2018, 10, 403.
- Davinelli, S.; Bertoglio, J.C.; Polimeni, A.; Scapagnini, G. Cytoprotective Polyphenols Against Chronological Skin Aging and Cutaneous Photodamage. Curr. Pharm. Des. 2018, 24, 99–105.
- Tundis, R.; Loizzo, M.R.; Bonesi, M.; Menichini, F. Potential role of natural compounds against skin aging. Curr. Med. Chem. 2015, 22, 1515–1538.
- Mukherjee, P.K.; Maity, N.; Nema, N.K.; Sarkar, B.K. Bioactive compounds from naturalresources against skin aging. Phytomedicine 2011, 19, 64–73.
- Działo, M.; Mierziak, J.; Korzun, U.; Preisner, M.; Szopa, J.; Kulma, A. The Potential of Plant Phenolics in Prevention and Therapy of Skin Disorders. Int. J. Mol. Sci. 2016, 17, 160.
- Dunaway, S.; Odin, R.; Zhou, L.; Ji, L.; Zhang, Y.; Kadekaro, A.L. Natural Antioxidants: Multiple Mechanisms to Protect Skin from Solar Radiation. Front. Pharmacol. 2018, 9, 392.
- Potapovich, A.I.; Lulli, D.; Fidanza, P.; Kostyuk, V.A.; De Luca, C.; Pastore, S.; Korkina, L.G. Plant polyphenols differentially modulate inflammatory responses of human keratinocytes by interfering with activation of transcription factors NFκB and AhR and EGFR-ERK pathway. Toxicol. Appl. Pharmacol. 2011, 255, 138–149.
- Mantena, S.K.; Katiyar, S.K. Grape seed proanthocyanidins inhibit UV-radiation-induced oxidative stress and activation of MAPK and NF-kappaB signaling in human epidermal keratinocytes. Free Radic. Biol. Med. 2006, 40, 1603–1614.
- Huang, Y.; Li, W.; Su, Z.Y.; Kong, A.N. The complexity of the Nrf2 pathway: Beyond the antioxidant response. J. Nutr. Biochem. 2015, 26, 1401–1413.
- Shindo, Y.; Witt, E.; Han, D.; Epstein, W.; Packer, L. Enzymic and non-enzymic antioxidants in epidermis and dermis of human skin. J. Invest. Dermatol. 1994, 102, 122–124.
- Bissett, D.L.; Oblong, J.E.; Berge, C.A. Niacinamide: A B vitamin that improves aging facial skin appearance. Dermatol. Surg. 2005, 31, 860–865.
- Rovito, H.A.; Oblong, J.E. Nicotinamide preferentially protects glycolysis in dermal fibroblasts under oxidative stress conditions. Br. J. Dermatol. 2013, 169, 15–24.
- Park, J.; Halliday, G.M.; Surjana, D.; Damian, D.L. Nicotinamide prevents ultraviolet radiation-induced cellular energy loss. Photochem. Photobiol. 2010, 86, 942–948.
- Surjana, D.; Halliday, G.M.; Martin, A.J.; Moloney, F.J.; Damian, D.L. Oral nicotinamide reduces actinic keratoses in phase II double-blinded randomized controlled trials. J. Invest. Dermatol. 2012, 132, 1497–1500.
- Damian, D.L.; Patterson, C.R.; Stapelberg, M.; Park, J.; Barnetson, R.S.; Halliday, G.M. UV radiation-induced immunosuppression is greater in men and prevented by topical nicotinamide. J. Invest. Dermatol. 2008, 128, 447–454.
- Damian, D.L. Photoprotective effects of nicotinamide. Photochem. Photobiol. Sci. 2010, 9, 578–585.
- Forbat, E.; Al-Niaimi, F.; Ali, F.R. Use of nicotinamide in dermatology. Clin. Exp. Dermatol. 2017, 42, 137–144.
- Li, J.; Bonkowski, M.S.; Moniot, S.; Zhang, D.; Hubbard, B.P.; Ling, A.J.; Rajman, L.A.; Qin, B.; Lou, Z.; Gorbunova, V.; et al. A conserved NAD(+) binding pocket that regulates protein-protein interactions during aging. Science 2017, 355, 1312–1317.
- Zouboulis, C.C.; Makrantonaki, E. Clinical aspects and molecular diagnostics of skin aging. Clin. Dermatol. 2011, 29, 3–14.
- Wu, Y.; Zheng, X.; Xu, X.G.; Li, Y.H.; Wang, B.; Gao, X.H.; Chen, H.D.; Yatskayer, M.; Oresajo, C. Protective effects of a topical antioxidant complex containing vitamins C and Enand ferulic acid against ultraviolet irradiation-induced photodamage in Chinese women. J. Drugs Dermatol. 2013, 12, 464–468.
- Murray, J.C.; Burch, J.A.; Streilein, R.D.; Iannacchione, M.A.; Hall, R.P.; Pinnell, S.R. A topical antioxidant solution containing vitamins C and E stabilized by ferulic acid provides protection for human skin against damage caused by ultraviolet irradiation. J. Am. Acad. Dermatol. 2008, 59, 418–425.
- Lin, F.H.; Lin, J.Y.; Gupta, R.D.; Tournas, J.A.; Burch, J.A.; Selim, M.A.; Monteiro-Riviere, N.A.; Grichnik, J.M.; Zielinski, J.; Pinnell, S.R. Ferulic acid stabilizes a solution of vitamins C and E and doubles its photoprotection of skin. J. Invest. Dermatol. 2005, 125, 826–832.
- Gupta, R.; Dixon, K.M.; Deo, S.S.; Holliday, C.J.; Slater, M.; Halliday, G.M.; Reeve, V.E.; Mason, R.S. Photoprotection by 1,25-dihydroxyvitamin D3 is associated with an increase in p53 and a decrease in nitric oxide products. J. Invest. Dermatol. 2007, 127, 707–715.
- Dixon, K.M.; Tongkao-On, W.; Sequeira, V.B.; Carter, S.E.; Song, E.J.; Rybchyn, M.S.; Gordon-Thomson, C.; Mason, R.S. Vitamin D and death by sunshine. Int. J. Mol. Sci. 2013, 14, 1964–1977.
- Song, E.J.; Gordon-Thomson, C.; Cole, L.; Stern, H.; Halliday, G.M.; Damian, D.L.; Reeve, V.E.; Mason, R.S. 1α,25-Dihydroxyvitamin D3 reduces several types of UV-induced DNA damage and contributes to photoprotection. J. Steroid Biochem. Mol. Biol. 2013, 136, 131–138.
- Bikle, D.D.; Elalieh, H.; Welsh, J.; Oh, D.; Cleaver, J.; Teichert, A. Protective role of vitamin D signaling in skin cancer formation. J. Steroid Biochem. Mol. Biol. 2013, 136, 271–279.
- Demetriou, S.K.; Ona-Vu, K.; Teichert, A.E.; Cleaver, J.E.; Bikle, D.D.; Oh, D.H. Vitamin D receptor mediates DNA repair and is UV inducible in intact epidermis but not in cultured keratinocytes. J. Invest. Dermatol. 2012, 132, 2097–2100.
- Jiang, Y.J.; Teichert, A.E.; Fong, F.; Oda, Y.; Bikle, D.D. 1α,25(OH)2-dihydroxyvitamin D3/VDR protects the skin from UVB-induced tumor formation by interacting with the β-catenin pathway. J. Steroid Biochem. Mol. Biol. 2013, 136, 229–232.
- Gordon-Thomson, C.; Gupta, R.; Tongkao-on, W.; Ryan, A.; Halliday, G.M.; Mason, R.S. 1α,25 dihydroxyvitamin D3 enhances cellular defences against UV-induced oxidative and other forms of DNA damage in skin. Photochem. Photobiol. Sci. 2012, 11, 1837–1847.
- Slominski, A.T.; Kim, T.K.; Shehabi, H.Z.; Semak, I.; Tang, E.K.; Nguyen, M.N.; Benson, H.A.; Korik, E.; Janjetovic, Z.; Chen, J.; et al. In vivo evidence for a novel pathway of vitamin D3 metabolism initiated by P450scc and modified by CYP27B1. FASEB J. 2012, 26, 3901–3915.
- Slominski, A.T.; Kim, T.K.; Shehabi, H.Z.; Tang, E.K.; Benson, H.A.; Semak, I.; Lin, Z.; Yates, C.R.; Wang, J.; Li, W.; et al. In vivo production of novel vitamin D2 hydroxy-derivatives by human placentas, epidermal keratinocytes, Caco-2 colon cells and the adrenal gland. Mol. Cell Endocrinol. 2014, 383, 181–192.
- Slominski, A.T.; Zmijewski, M.A.; Semak, I.; Sweatman, T.; Janjetovic, Z.; Li, W.; Zjawiony, J.K.; Tuckey, R.C. Sequential metabolism of 7-dehydrocholesterol to steroidal 5,7-dienes in adrenal glands and its biological implication in the skin. PLoS ONE 2009, 4, e4309.
- Slominski, A.T.; Kim, T.K.; Chen, J.; Nguyen, M.N.; Li, W.; Yates, C.R.; Sweatman, T.; Janjetovic, Z.; Tuckey, R.C. Cytochrome P450scc-dependent metabolism of 7-dehydrocholesterol in placenta and epidermal keratinocytes. Int. J. Biochem. Cell Biol. 2012, 44, 2003–2018.
- Chen, J.; Wang, J.; Kim, T.K.; Tieu, E.W.; Tang, E.K.; Lin, Z.; Kovacic, D.; Miller, D.D.; Postlethwaite, A.; Tuckey, R.C.; et al. Novel vitamin D analogs as potential therapeutics: Metabolism, toxicity profiling, and antiproliferative activity. Anticancer Res. 2014, 34, 2153–2163.
- Slominski, A.T.; Janjetovic, Z.; Kim, T.K.; Wasilewski, P.; Rosas, S.; Hanna, S.; Sayre, R.M.; Dowdy, J.C.; Li, W.; Tuckey, R.C. Novel non-calcemic secosteroids that are produced by human epidermal keratinocytes protect against solar radiation. J. Steroid Biochem. Mol. Biol. 2015, 148, 52–63.
- Oda, K.; Arakawa, H.; Tanaka, T.; Matsuda, K.; Tanikawa, C.; Mori, T.; Nishimori, H.; Tamai, K.; Tokino, T.; Nakamura, Y.; et al. p53AIP1, a potential mediator of p53-dependent apoptosis, and its regulation by Ser-46-phosphorylated p53. Cell 2000, 102, 849–862.
- Tuckey, R.C.; Cheng, C.Y.S.; Slominski, A.T. The serum vitamin D metabolome: What we know and what is still to discover. J. Steroid Biochem. Mol. Biol. 2019, 186, 4–21.
- Kim, T.K.; Lin, Z.; Li, W.; Reiter, R.J.; Slominski, A.T. N1-Acetyl-5-Methoxykynuramine (AMK) is produced in the human epidermis and shows antiproliferative effects. Endocrinology 2015, 156, 1630–1636.
- Kim, T.K.; Lin, Z.; Tidwell, W.J.; Li, W.; Slominski, A.T. Melatonin and its metabolites accumulate in the human epidermis in vivo and inhibit proliferation and tyrosinase activity in epidermal melanocytes in vitro. Mol. Cell Endocrinol. 2015, 404, 1–8.
- Jockers, R.; Delagrange, P.; Dubocovich, M.L.; Markus, R.P.; Renault, N.; Tosini, G.; Cecon, E.; Zlotos, D.P. Update on melatonin receptors: IUPHAR Review 20. Br. J. Pharmacol. 2016, 173, 2702–2725.
- Slominski, R.M.; Reiter, R.J.; Schlabritz-Loutsevitch, N.; Ostrom, R.S.; Slominski, A.T. Melatonin membrane receptors in peripheral tissues: Distribution and functions. Mol. Cell Endocrinol. 2012, 351, 152–166.
- Slominski, A.; Pisarchik, A.; Zbytek, B.; Tobin, D.J.; Kauser, S.; Wortsman, J. Functional activity of serotoninergic and melatoninergic systems expressed in the skin. J. Cell Physiol. 2003, 196, 144–153.
- Slominski, A.T.; Hardeland, R.; Zmijewski, M.A.; Slominski, R.M.; Reiter, R.J.; Paus, R. Melatonin: A cutaneous perspective on its production, metabolism, and functions. J. Invest. Dermatol. 2018, 138, 490–499.
- Majidinia, M.; Reiter, R.J.; Shakouri, S.K.; Yousefi, B. The role of melatonin, a multitasking molecule, in retarding the processes of ageing. Ageing Res. Rev. 2018, 47, 198–213.
- Reiter, R.J.; Mayo, J.C.; Tan, D.X.; Sainz, R.M.; Alatorre-Jimenez, M.; Qin, L. Melatonin as an antioxidant: Under promises but over delivers. J. Pineal Res. 2016, 61, 253–278.
- Galano, A.; Tan, D.X.; Reiter, R.J. Melatonin: A Versatile Protector against Oxidative DNA Damage. Molecules 2018, 23, 530.
- Rodriguez, C.; Mayo, J.C.; Sainz, R.M.; Antolín, I.; Herrera, F.; Martín, V.; Reiter, R.J. Regulation of antioxidant enzymes: A significant role for melatonin. J. Pineal Res. 2004, 36, 1–9.
- Fischer, T.W.; Scholz, G.; Knöll, B.; Hipler, U.C.; Elsner, P. Melatonin suppresses reactive oxygen species in UV-irradiated leukocytes more than vitamin C and trolox. Skin Pharmacol. Appl. Skin Physiol. 2002, 15, 367–373.
- Janjetovic, Z.; Nahmias, Z.P.; Hanna, S.; Jarrett, S.G.; Kim, T.K.; Reiter, R.J.; Slominski, A.T. Melatonin and its metabolites ameliorate ultraviolet B-induced damage in human epidermal keratinocytes. J. Pineal Res. 2014, 57, 90–102.
- Janjetovic, Z.; Jarrett, S.G.; Lee, E.F.; Duprey, C.; Reiter, R.J.; Slominski, A.T. Melatonin and its metabolites protect human melanocytes against UVB-induced damage: Involvement of NRF2-mediated pathways. Sci. Rep. 2017, 7, 1274.
- Rezzani, R.; Rodella, L.F.; Favero, G.; Damiani, G.; Paganelli, C.; Reiter, R.J. Attenuation of ultraviolet A-induced alterations in NIH3T3 dermal fibroblasts by melatonin. Br. J. Dermatol. 2014, 170, 382–391.
- Lee, K.S.; Lee, W.S.; Suh, S.I.; Kim, S.P.; Lee, S.R.; Ryoo, Y.W.; Kim, B.C. Melatonin reduces ultraviolet-B induced cell damages and polyamine levels in human skin fibroblastsin culture. Exp. Mol. Med. 2003, 35, 263–268.
- Manchester, L.C.; Coto-Montes, A.; Boga, J.A.; Andersen, L.P.; Zhou, Z.; Galano, A.; Vriend, J.; Tan, D.X.; Reiter, R.J. Melatonin: An ancient molecule that makes oxygen metabolically tolerable. J. Pineal. Res. 2015, 59, 403–419.
- Mayo, J.C.; Sainz, R.M.; Antoli, I.; Herrera, F.; Martin, V.; Rodriguez, C. Melatonin regulation of antioxidant enzyme gene expression. Cell Mol. Life Sci. 2002, 59, 1706–1713.
- Sliwinski, T.; Rozej, W.; Morawiec-Bajda, A.; Morawiec, Z.; Reiter, R.; Blasiak, J. Protective action of melatonin against oxidative DNA damage: Chemical inactivation versus base-excision repair. Mutat. Res. 2007, 634, 220–227.
- Skobowiat, C.; Brożyna, A.A.; Janjetovic, Z.; Jeayeng, S.; Oak, A.S.W.; Kim, T.K.; Panich, U.; Reiter, R.J.; Slominski, A.T. Melatonin and its derivatives counteract the ultraviolet B radiation-induced damage in human and porcine skin ex vivo. J. Pineal Res. 2018, 65, 12501.
- Santoro, R.; Marani, M.; Blandino, G.; Muti, P.; Strano, S. Melatonin triggers p53Ser phosphorylation and prevents DNA damage accumulation. Oncogene 2012, 31, 2931–2942.
- Fischer, T.W.; Zmijewski, M.A.; Wortsman, J.; Slominski, A. Melatonin maintains mitochondrial membrane potential and attenuates activation of initiator (casp-9) and effector caspases (casp-3/casp-7) and PARP in UVR-exposed HaCaT keratinocytes. J. Pineal Res. 2008, 44, 397–407.
- Kleszczyński, K.; Bilska, B.; Stegemann, A.; Flis, D.J.; Ziolkowski, W.; Pyza, E.; Luger, T.A.; Reiter, R.J.; Böhm, M.; Slominski, A.T. Melatonin and Its Metabolites Ameliorate UVR-Induced Mitochondrial Oxidative Stress in Human MNT-1 Melanoma Cells. Int. J. Mol. Sci. 2018, 19, 3786.
- Milani, M.; Sparavigna, A. Antiaging efficacy of melatonin-based day and night creams: A randomized, split-face, assessor-blinded proof-of-concept trial. Clin. Cosmet. Investig. Dermatol. 2018, 11, 51–57.
- Sagan, D.; Stepniak, J.; Gesing, A.; Lewinski, A.; Karbownik-Lewinska, M. Melatonin reverses the enhanced oxidative damage to membrane lipids and improves skin biophysical characteristics in former-smokers—A study in postmenopausal women. Ann. Agric. Environ. Med. 2017, 24, 659–666.
- Manna, P.R.; Slominski, A.T.; King, S.R.; Stetson, C.L.; Stocco, D.M. Synergistic activation of steroidogenic acute regulatory protein expression and steroid biosynthesis by retinoids: Involvement of cAMP/PKA signaling. Endocrinology 2014, 155, 576–591.
More
