Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Amina Yu and Version 1 by Vinay Aseri.
Soil is a compound mixture and a non-renewable natural resource, as it can only be restored on a geological timescale. It can be easily defined as the loose inorganic or organic matter of the surface that assists as a natural habitat for terrestrial plants. Heavy metals are very hazardous to the environment and living things.
- heavy metal
- remediation
- environmental
- phytoremediation
- nanotechnology
1. Heavy Metals Contamination and Toxicity in Soil Ecosystem
Heavy metal accumulation in the soil is hazardous to the environment and human health, and well-known harmful contaminants have devastating effects on the biological circulation of terrestrial species with variations in the structural composition of nucleic acids, proteins and osmotic balance [32][1]. Although several remediation techniques such as hardening/stabilization (S/S), soil leaching, electrokinetic remediation, and chemical oxidation/reduction are used to fix, remove, or detoxify HMs in the soil, these traditional approaches do not result in overall sustainability [33][2].
Metal toxicity not only affects aquatic organisms, but also harmful to soil flora, plants, animals, and humans as well. Oxidative stress results in damage to cell morphology and inhibits cytoplasmic enzymes [34][3]. Usually, these metals exist in nature individually or in grouping with other elements, but anthropogenic activity increases their concentrations in the environment [35][4]. Since HMs are water-soluble, they are mainly soluble in solutions. This makes it difficult to remove by physical and chemical separation processes in the soil [36][5]. Solubility of HMs is determined by their chemical morphology in the environment. So, for improving the remediation efficiency of microbial fuel cell (MFC), appropriate methods for converting HMs into easy-to-move forms (such as acid-soluble fractions) are needed. Some research has used auxiliary reagents like small-molecule organic acids (citric acid, CA; and acetic acid, HAc), inorganic acids (HCl, HNO3), and synthetic chelating agents (ethylenediaminetetraacetic acid, EDTA) [37,38][6][7].
These chemicals help in desorbing and dissolving HMs in the soil, allowing them to move around more freely. Synthetic chelating chemicals pose a risk since polymer chelates migrate slowly in electric fields and secondary ecological settings [39][8]. This sItudy used two small-molecule organic acids (CA, HAc) that are commonly available, reasonably inexpensive, and ecologically benign, as well as a mineral acid (HCl) [39][8]. The rate of faster improvement of the industrial sector has raised the HM contamination problem, like a hike in manufacturing purposes for other metals. Heavy metals like Cd, Pb, As, Cr, Cu, and Zn are mainly used in industry and agriculture. Small amounts of these metals are lethal.
Although these metals are present naturally in the environment, tampering occurs when there are large amounts of these metals on land due to continuous mining as well as smelting [7,40][9][10]. As industrialization progresses and the natural biogeochemical cycle are disrupted, the issue of HM contamination becomes more and more serious. Heavy metals, unlike biological compounds, seldom biodegrade and hence gather in the environment. Accumulation of HMs takes place in the tissues of an organism (bioaccumulation), and their concentrations increase as they transition from low to high trophic levels (biomagnification). Heavy metals in the soil have toxicological consequences on soil microorganisms, which leads to reduced numbers and activity [1][11].
2. Sustainable Remediation Strategies
2.1. Nano-Bio Remediation of Heavy Metals: Application and Implications
In the modern era, the usage of nanoparticles (NPs) in industry, medicine, agriculture and cosmetics has increased significantly [67,68,69,70,71,72,73,74,75,76,77,78,79,80][12][13][14][15][16][17][18][19][20][21][22][23][24][25]. Materials with at least one dimension smaller than 100 nm are commonly referred to as NP. NPs with various particle sizes, shapes, as well as functions are produced as per requirement [81][26]. Compared to conventional materials, NPs possess a lot of advantages, including increased surface activity, extra reactive sites on the surface, increased catalytic efficiency, and special optical as well as magnetic properties [82,83,84][27][28][29]. The environmental impact of NPs is discussed in previous research: Hao et al. showed that even at 10 mg L−1, rice contained endophytic fungi sensitive to carbon-based NPs [85][30]. It is reported that exposure to Ag NPs at a dose of 50 mg kg−1 adversely affects the biomass and quality of peanuts [86][31]. In addition, low dosages (5 and 50 mg kg−1) of NiO had no effect on the survival, reproduction as well as rate of growth of adult earthworms, whereas high dosages (200 and 500 mg/kg) expressly affects physiological and biochemical effects and turned out to be the endpoint [80][25]. As many field studies (pilot and life-size) and laboratory studies show, the use of nanotechnology for water remediation, used for drinking purification and pollution control, is very favorable.
There are many reviews of applications based on nanotechnology. However, in order to further elucidate its significance as well as guide development, it is necessary to directly compare existing therapeutic methods with new approaches using nanotechnology. In this review, the effectiveness of effectiveness of nanotechnology and old technologies for water purification as well as environmental improvement to provide industries, researchers, and policy makers with insight into the status of water purification methods, are compared. Contaminants were classified into a wide range of classes and the most gainful methods were compared in each class described in the literature. A case study is also presented that directly compared conventional techniques to nanotechnology-based techniques for similar contaminants. Nanotechnology-based methods are generally considered costly, but many of these offer inexpensive and more operative options to traditional technologies. Additionally, nano-based technologies can be critical to complying with progressively stringent water quality standards, especially to remove new and low-concentration pollutants [87][32].
All latest techniques as well as industries in pharmaceutical departments are interfaced by nanotechnology [88[33][34],89], textile industry [90][35], electrical industry [91[36][37],92], mechanical technology, and environment-related industries. Nanotechnology is considered as the synthesis and processing of nanoscale materials [93][38]. Their small size is the only cause that increases the cost of these materials [94][39]. Due to their small size, the surface area to volume ratio is significantly improved and the bandgap is clean and wide. As a result, their optical, physical, and electrical properties differ significantly from large volumes of material. Nanomaterials can be metal, semiconductors, or organic [95][40]. The generation of artificial NPs is attracting attention as an effective recovery method. In addition to other environmental uses, NPs can be inserted underground in the form of sediment to offer conditions for chemical recovery of pollutant columns and for use as adsorbents and catalysts in wastewater treatment processes. Its efficiency is based on its chemical composition, well-defined shape and high specific surface area. Today, many NP products are formulated to effectively remove contaminants. However, there is rising concern about the probable impacts in the secondary life cycle linked with production [96,97][41][42].
Biosynthetic NPs help green remediation in a variety of methods. Iron-based NPs should be used directly as a fixative. For example, nano zero ferrous iron (nZVI) by waste tea can be used to reduce Cr (VI) in soil. Iron oxide NPs made from leaf extract can steady Cd and As in soil by coprecipitation [98,99][43][44]. For additional data on the use of nZVI NPs as well as iron oxide, refer to the reader [100][45]. Green NPs indirectly aid in soil regeneration. Ag NPs mediated by plant extracts can promote plant growth by increasing soil pH value, nutrient bioavailability and water retention capacity [65,68,101][46][13][47]. Naturally benign nano-sized mineral built soil conditioners can be organized with feldspar and lime consuming a mild hydrothermal method [102][48]. Their use in NPs synthesis is attracting more and more attention. Summarizing the current report on the synthesis of NPs for environmental restoration with the help of plant extracts (bud system). NP recovery in vivo is attained through the occurrence of biomolecules contained in plant extracts.
The precise mechanism of this procedure is not yet fully understood, but amino acids, citric acid, phenol, sugar, membrane protein, tartaric acid, as well as functional groups (alcohols, aldehydes, amines, and ketones of carboxylic acids) also reduce and block reducing agents [67,103,104,105][12][49][50][51].
2.2. Biochar Based Sustainable Amelioration of Soil
In recent times, biochar has gained increasing courtesy as an environmentally friendly tactic, especially as a weather protection strategy [106][52] (Figure 1). Biochar is considered as a carbon-rich, fine-grained, porous material formed by the thermal decay of biomass at relatively low temperatures under oxygen-restricted conditions (<700 C) [76,79,107][21][24][53]. It is also considered as a predominantly stable and stubborn organic carbon (C) compound formed when biomass (raw material) has a temperature typically between 300 °C and 100 °C at low oxygen levels.

Biochar is rich in carbon, porous, and has a large exact surface area, and this exact structure has been shown to be capable of enhancing soil moisture and nutrient retention [108][57]. Biochars range from crop residues (corn stalks, rice straw, rice husks, rapeseed stalks, etc.), grass, wood, sewage sludge, anaerobic digests, and animal excrement (poultry litter, pig manure, etc.). It can be produced by heat treatment of biological waste, and chicken manure) [109,110,111][58][59][60]. Biochar interacts with HMs in many ways. Complex formation of the outer sphere, complex of the inner sphere, electrostatic interactions, surficial precipitation, and exchange of ion are potential mechanisms of metal fixation [20,112][61][62]. A new trend has appeared in biochar pyrolysis as well as post-pyrolysis transformation plans to increase the metal binding capacity of biochar adsorbents (Table 1) [113][63].
2.3. Fly Ash- Industrial-Based Materials for Sustainable Remediation
Since they are mass-produced every year, industrial by-products are attracting attention. Reuse as a soil conditioner is a viable method to the sustainable use of these minimum value by-products. Fly ash from coal combustion is a distinctive byproduct of the coal industry. About 780 million tons are made yearly. The chemical composition of fly ash differs due to its dissimilar source and composition of burned coal, but all types include significant amounts of SiO2, Al2O3, Fe2O3, and CaO [73,116,117][18][56][64]. It has a similar mechanism of metal immobilization by oxides (i.e., lime and precipitation, surface complexion). The Bayer process in the alumina industry gives red mud as a by-product [118][65].
However, industrial waste can contain large amounts of poisonous metals as well as organic pollutants. When applied to the soil, these pollutants are out and can move over the long term, posing a danger to the environment [119][66]. Agriculture, livestock as well as food industries in particular are the major producers of organic waste. Sludge from the sugar industry alone accounts for 30 million tons worldwide [120][67]. In India alone, the paper industry, which uses large amounts of water and plant cellulose materials, produces 3.033 tons of bio-waste by-products annually from paper mills [121][68]. In fact, Asian countries have deprived reprocessing systems and faced more ecological problems. Industrial waste is composed of various harmful substances, especially HMs as well as other organic pollutants that affect the quality of the soil. Currently, more than 40% of bio-waste is landfilled, producing both carbon dioxide and methane [122][69]. Unlimited greenhouse gas creation from bio-waste landfills that endanger the environment and environmental issues require scientific intervention. As shown, the origin as well as industrial biowaste production can be manageable in a variety of ways, ultimately leading to agriculture. The wastes which are polluted dispose in open places that risk to the environment. Generally, pollutants can be separated into two subgroups: (i) organic and (ii) inorganic [66,123,124][70][71][72]. Currently, contaminated bio-waste is disposed of in vacancies, which are a major cause of environmental pollution, especially water and soil pollution. Therefore, restoration techniques are needed to manage some bio-waste [125][73] (Figure 2).
2.4. Employing Bioremediation for Remediation of Contaminated Soil
Biotechnology which utilizes plants’ potential to improve the health of the environment is called phytoremediation. Applied research shows that plants have the capability to eliminate and degrade a range of HM toxicants. Due to being cost effective, simple, sustainable, and being compatible with the environment it is considered as more aesthetic than traditional technologies. Remediation of large amounts of toxic groundwater and treatment of large volumes of diluted wastewater can be implemented in situ. Plants respond differently to metal adulteration in soil and should be divided into different groups depending on their response to metal adulteration in the rooting medium. Plants should be divided as accumulators, indicators, or excluders, dependent on the uptake and movement of metal into the ground by the plant [20][61]. The phytoremediation technique is simple, inexpensive, sustainable, companionable, environment friendly as well as one of the key contents of green technology.
Plants have the natural capability to break down the HMs through a variety of procedures such as bioaccumulation, translocation, and storage/decomposition of pollutants. Phytoremediation is 10 times more inexpensive than traditional technical approaches because it is field-based, photovoltaic, and can function with minimal post-installation maintenance [75,77,128][20][22][76]. Plants have been reported to be highly resistant to HM pollution without causing serious harm; these properties of plants suggest that they could be used to detoxify pollutants through novel approaches to agriculture and genetic engineering. Some of the plants have the natural capability to break down many awkward xenobiotic substances and are therefore considered “green livers” that serve as an essential source of absorption of environmentally harmful chemicals. Nature gives plants the excellent ability to defuse these poisonous elements in the growing matrix, whether in soil or water (Figure 3) [129][77].
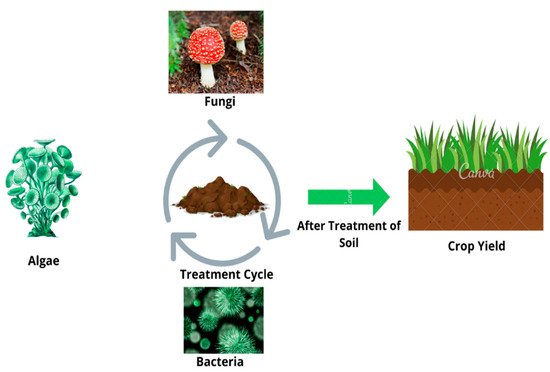
Figure 3. Bioremediation process showing the role of algae, fungi, and bacteria for the enhancement of soil health.
References
- Sivakumar, G.; Xu, J.; Thompson, R.W.; Yang, Y.; Randol-Smith, P.; Weathers, P.J. Integrated green algal technology for bioremediation and biofuel. Bioresour. Technol. 2012, 107, 1–9.
- Wang, L.; Rinklebe, J.; Tack, F.M.; Hou, D. A review of green remediation strategies for heavy metal contaminated soil. Soil Use Manag. 2021, 37, 936–963.
- Bulgariu, L.; Bulgariu, D. Enhancing biosorption characteristics of marine green algae (Ulva lactuca) for heavy metals removal by alkaline treatment. Bioprocess. Biotech. 2014, 4, 1.
- Bestawy, E.E.; Helmy, S.; Hussien, H.; Fahmy, M.; Amer, R. Bioremediation of heavy metal-contaminated effluent using optimized activated sludge bacteria. Appl. Water Sci. 2013, 3, 181–192.
- Volesky, B.; Naja, G. Biosorption technology: Starting up an enterprise. Int. J. Technol. Transf. Commer. 2007, 6, 196–211.
- Kim, K.J.; Kim, D.H.; Yoo, J.C.; Baek, K. Electrokinetic extraction of heavy metals from dredged marine sediment. Sep. Purif. Technol. 2011, 79, 164–169.
- Cameselle, C.; Pena, A. Enhanced electromigration and electro-osmosis for the remediation of an agricultural soil contaminated with multiple heavy metals. Process. Saf. Environ. Ptotection 2016, 104, 209–217.
- Lan, J.; Zhang, S.; Lin, H.; Li, T.; Xu, X.; Li, Y.; Gong, G. Efficiency of biodegradable EDDS, NTA and APAM on enhancing the phytoextraction of cadmium by Siegesbeckia orientalis L. grown in Cd-contaminated soils. Chemosphere 2013, 91, 1362–1367.
- Verma, R.K.; Sankhla, M.S.; Jadhav, E.B.; Parihar, K.; Awasthi, K.K. Phytoremediation of heavy metals extracted soil and aquatic environments: Current advances as well as emerging trends. Biointerface Res. Appl. Chem. 2021, 12, 5486–5509.
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. Mol. Clin. Environ. Toxicol. 2012, 101, 133–164.
- Khan, S.; Hesham, A.E.L.; Qiao, M.; Rehman, S.; He, J.Z. Effects of Cd and Pb on soil microbial community structure and activities. Environ. Sci. Pollut. Res. 2010, 17, 288–296.
- Awasthi, A.; Sharma, P.; Jangir, L.; Awasthi, G.; Awasthi, K.K.; Awasthi, K. Dose dependent enhanced antibacterial effects and reduced biofilm activity against Bacillus subtilis in presence of ZnO nanoparticles. Mater. Sci. Eng. C 2020, 113, 111021.
- Sankhla, M.S.; Kumar, R. Contaminant of Heavy Metals in Groundwater & Its Toxic Effects on Human Health & Environment. Int. J. Environ. Sci. Nat. Res. 2019, 18, 555996.
- Ishii, S.; Bell, J.; Marshall, F.J.E.P. Phytotoxic risk assessment of ambient air pollution on agricultural crops in Selangor State, Malaysia. Environ. Pollut. 2007, 150, 267–279.
- Rafique, T.; Naseem, S.; Usmani, T.H.; Bashir, E.; Khan, F.A.; Bhanger, M.I. Geochemical factors controlling the occurrence of high fluoride groundwater in the Nagar Parkar area, Sindh, Pakistan. J. Hazard. Mater. 2009, 171, 424–430.
- Awasthi, G.; Singh, T.; Tiwari, Y.; Awasthi, A.; Tripathi, R.D.; Shrivastava, S.; Awasthi, K.K. A review on nanotechnological interventions for plant growth and production. Mater. Today Proc. 2020, 31, 685–693.
- Awasthi, G.; Kumar, A.; Awasthi, K.K.; Singh, A.P.; Srivastva, S.; Vajpayee, P.; Tripathi, R.D. Green synthesis of nanoparticles: An emerging phytotechnology. In Green Technologies and Environmental Sustainability; Springer: Cham, Switzerland, 2017; pp. 339–363.
- Yadav, H.; Kumar, R.; Sankhla, M.S. Residues of pesticides and heavy metals in crops resulting in toxic effects on living organism. J. Seybold Rep. ISSN NO 2020, 1533, 9211.
- Srivastava, S. Arsenic in Drinking Water and Food; Springer: Berlin/Heidelberg, Germany, 2020.
- Sankhla, M.S.; Kumari, M.; Nandan, M.; Kumar, R.; Agrawal, P. Heavy metals contamination in water and their hazardous effect on human health—A review. Int. J. Curr. Microbiol. App. Sci. 2016, 5, 759–766.
- Katare, P.Y.; Sankhla, M.S.; Singhal, M.; Ekta, B.; Jadhav, K.P.; Bhagyashri, T.N.; Bhardwaj, L. Microplastics in aquatic environments: Sources, ecotoxicity, detection & remediation. Biointerface Res. Appl. Chem. 2021, 12, 3407–3428.
- Sankhla, M.S.; Kumari, M.; Sharma, K.; Kushwah, R.S.; Kumar, R. Heavy metal pollution of Holy River Ganga: A review. Int. J. Res. 2018, 5, 421–436.
- Parihar, K.; Kumar, R.; Sankhla, M.S. Impact of Heavy Metals on Survivability of Earthworms. Int. Med.-Leg. Report. J. 2019, 26, 51–57.
- Verma, R.K.; Sankhla, M.S.; Rathod, N.V.; Sonone, S.S.; Parihar, K.; Singh, G.K. Eradication of fatal textile industrial dyes by wastewater treatment. Biointerface Res. Appl. Chem. 2021, 12, 567–587.
- Adeel, M.; Ma, C.; Ullah, S.; Rizwan, M.; Hao, Y.; Chen, C.; Jilani, G.; Shakoor, N.; Li, M.; Wang, L.; et al. Exposure to nickel oxide nanoparticles insinuates physiological, ultrastructural and oxidative damage: A life cycle study on Eisenia fetida. Environ. Pollut. 2019, 254, 113032.
- Adeel, M.; Tingting, J.; Hussain, T.; He, X.; Ahmad, M.A.; Irshad, M.K.; Shakoor, N.; Zhang, P.; Changjian, X.; Hao, Y.; et al. Bioaccumulation of ytterbium oxide nanoparticles insinuate oxidative stress, inflammatory, and pathological lesions in ICR mice. Environ. Sci. Pollut. Res. 2020, 27, 32944–32953.
- Wang, Y.; Jiang, F.; Ma, C.; Rui, Y.; Tsang, D.C.; Xing, B. Effect of metal oxide nanoparticles on amino acids in wheat grains (Triticum aestivum) in a life cycle study. J. Environ. Manag. 2019, 241, 319–327.
- Yang, J.; Cao, W.; Rui, Y. Interactions between nanoparticles and plants: Phytotoxicity and defense mechanisms. J. Plant Interact. 2017, 12, 158–169.
- Yang, J.; Jiang, F.; Ma, C.; Rui, Y.; Rui, M.; Adeel, M.; Cao, W.; Xing, B. Alteration of crop yield and quality of wheat upon exposure to silver nanoparticles in a life cycle study. J. Agric. Food Chem. 2018, 66, 2589–2597.
- Hao, Y.; Ma, C.; White, J.C.; Adeel, M.; Jiang, R.; Zhao, Z.; Rao, Y.; Chen, G.; Rui, Y.; Xing, B. Carbon-based nanomaterials alter the composition of the fungal endophyte community in rice (Oryza sativa L.). Environ. Sci. Nano 2020, 7, 2047–2060.
- Rui, M.; Ma, C.; Tang, X.; Yang, J.; Jiang, F.; Pan, Y.; Xiang, Z.; Hao, Y.; Rui, Y.; Cao, W.; et al. Phytotoxicity of silver nanoparticles to peanut (Arachis hypogaea L.): Physiological responses and food safety. ACS Sustain. Chem. Eng. 2017, 5, 6557–6567.
- Adeleye, A.S.; Conway, J.R.; Garner, K.; Huang, Y.; Su, Y.; Keller, A.A. Engineered nanomaterials for water treatment and remediation: Costs, benefits, and applicability. Chem. Eng. J. 2016, 286, 640–662.
- Jafari, S.M.; McClements, D.J. Nanotechnology approaches for increasing nutrient bioavailability. Adv. Food Nutr. Res. 2017, 81, 1–30.
- Caracciolo, G.; Vali, H.; Moore, A.; Mahmoudi, M. Challenges in molecular diagnostic research in cancer nanotechnology. Nano Today 2019, 27, 6–10.
- Gashti, M.P.; Pakdel, E.; Alimohammadi, F. Nanotechnology-based coating techniques for smart textiles. In Active Coatings for Smart Textiles; Woodhead Publishing: Sawston, UK, 2016; pp. 243–268.
- Contreras, J.; Rodriguez, E.A.; Taha-Tijerina, J. Nanotechnology applications for electrical transformers—A review. Electr. Power Syst. Res. 2017, 143, 573–584.
- Contreras, J.E.; Rodriguez, E.A. Nanostructured insulators—A review of nanotechnology concepts for outdoor ceramic insulators. Ceram. Int. 2017, 43, 8545–8550.
- Hassan, D.; Khalil, A.T.; Solangi, A.R.; El-Mallul, A.; Shinwari, Z.K.; Maaza, M. Physiochemical properties and novel biological applications of Callistemon viminalis-mediated α-Cr2O3 nanoparticles. Appl. Organomet. Chem. 2019, 33, e5041.
- Hassan, D.; Khalil, A.T.; Saleem, J.; Diallo, A.; Khamlich, S.; Shinwari, Z.K.; Maaza, M. Biosynthesis of pure hematite phase magnetic iron oxide nanoparticles using floral extracts of Callistemon viminalis (bottlebrush): Their physical properties and novel biological applications. Artif. Cells Nanomed. Biotechnol. 2018, 46, 693–707.
- Khalil, A.T.; Ovais, M.; Ullah, I.; Ali, M.; Shinwari, Z.K.; Hassan, D.; Maaza, M. Sageretia thea (Osbeck.) modulated biosynthesis of NiO nanoparticles and their in vitro pharmacognostic, antioxidant and cytotoxic potential. Artif. Cells Nanomed. Biotechnol. 2018, 46, 838–852.
- Jin, X.; Yu, B.; Lin, J.; Chen, Z. Integration of biodegradation and nano-oxidation for removal of PAHs from aqueous solution. ACS Sustain. Chem. Eng. 2016, 4, 4717–4723.
- Prasad, K.S.; Gandhi, P.; Selvaraj, K. Synthesis of green nano iron particles (GnIP) and their application in adsorptive removal of As (III) and As (V) from aqueous solution. App. Surf. Sci. 2014, 317, 1052–1059.
- Lin, J.; He, F.; Su, B.; Sun, M.; Owens, G.; Chen, Z. The stabilizing mechanism of cadmium in contaminated soil using green synthesized iron oxide nanoparticles under long-term incubation. J. Hazard. Mater. 2019, 379, 120832.
- Zhan, J.; Huang, H.; Yu, H.; Zhang, X.; Zheng, Z.; Wang, Y.; Liu, T.; Li, T. The combined effects of Cd and Pb enhanced metal binding by root cell walls of the phytostabilizer Athyrium wardii (Hook.). Environ. Pollut. 2020, 258, 113663.
- Wang, Y.; O’Connor, D.; Shen, Z.; Lo, I.M.; Tsang, D.C.; Pehkonen, S.; Pu, S.; Hou, D. Green synthesis of nanoparticles for the remediation of contaminated waters and soils: Constituents, synthesizing methods, and influencing factors. J. Clean. Prod. 2019, 226, 540–549.
- Song, T.S.; Zhang, J.; Hou, S.; Wang, H.; Zhang, D.; Li, S.; Xie, J. In situ electrokinetic remediation of toxic metal-contaminated soil driven by solid phase microbial fuel cells with a wheat straw addition. J. Chem. Technol. Biotechnol. 2018, 93, 2860–2867.
- Das, P.; Barua, S.; Sarkar, S.; Karak, N.; Bhattacharyya, P.; Raza, N.; Kim, K.H.; Bhattacharya, S.S. Plant extract–mediated green silver nanoparticles: Efficacy as soil conditioner and plant growth promoter. J. Hazard. Mater. 2018, 346, 62–72.
- Zhang, Y.; Wei, J.; Zhu, Y.; George-Ufot, G. Untangling the relationship between corporate environmental performance and corporate financial performance: The double-edged moderating effects of environmental uncertainty. J. Clean. Prod. 2020, 263, 121584.
- Al-Shnani, F.; Al-Haddad, T.; Karabet, F.; Allaf, A.W. Chitosan loaded with silver nanoparticles, CS-AgNPs, using Thymus syriacus, wild mint, and rosemary essential oil extracts as reducing and capping agents. J. Phys. Org. Chem. 2017, 30, e3680.
- Selvan, D.A.; Mahendiran, D.; Kumar, R.S.; Rahiman, A.K. Garlic, green tea and turmeric extracts-mediated green synthesis of silver nanoparticles: Phytochemical, antioxidant and in vitro cytotoxicity studies. J. Photochem. Photobiol. B Biol. 2018, 180, 243–252.
- Weng, X.; Huang, L.; Chen, Z.; Megharaj, M.; Naidu, R. Synthesis of iron-based nanoparticles by green tea extract and their degradation of malachite. Ind. Crop. Prod. 2013, 51, 342–347.
- Lehmann, J.; Joseph, S. Biochar for Environmental Management: An Introduction; Routledge: Abington-on-Thames, UK, 2015.
- Mesa, A.C.; Spokas, K.A. Impacts of biochar (black carbon) additions on the sorption and efficacy of herbicides. Herbic. Environ. 2011, 13, 315–340.
- Bio Char Plant—Bing Images Rice Husk Biochar with Beneficial Microbes: A Promising Agricultural Inoculant and Soil Ameliorant—Research Outreach. Available online: https://researchoutreach.org/articles/rice-husk-biochar-agricultural-inoculant-soil-ameliorant/ (accessed on 8 December 2021).
- Biochar Compost FTW|Food|Forest|Garden. Available online: foodforestgarden.org (accessed on 8 December 2021).
- World-Biochar Headlines-02-2019 Biochar Project, Biochar Australia. Available online: http://biocharproject.org/world-biochar-headlines/world-biochar-headlines-02-2019/ (accessed on 8 December 2021).
- Santos, R.A.C. Desenvolvimento de Método para Determinação de Agrotóxicos (Perturbadores Endócrinos) em Água; UFS: São Cristóvão, Brazil, 2016; Available online: https://ri.ufs.br/handle/riufs/6050 (accessed on 8 December 2021).
- El-Naggar, A.; Lee, S.S.; Rinklebe, J.; Farooq, M.; Song, H.; Sarmah, A.K.; Zimmerman, A.R.; Ahmad, M.; Shaheen, S.M.; Ok, Y.S. Biochar application to low fertility soils: A review of current status, and future prospects. Geoderma 2019, 337, 536–554.
- Palansooriya, K.N.; Yang, Y.; Tsang, Y.F.; Sarkar, B.; Hou, D.; Cao, X.; Meers, E.; Rinklebe, J.; Kim, K.H.; Ok, Y.S. Occurrence of contaminants in drinking water sources and the potential of biochar for water quality improvement: A review. Crit. Rev. Environ. Sci. Technol. 2020, 50, 549–611.
- Shaheen, S.M.; Niazi, N.K.; Hassan, N.E.; Bibi, I.; Wang, H.; Tsang, D.C.; Ok, Y.S.; Bolan, N.; Rinklebe, J. Wood-based biochar for the removal of potentially toxic elements in water and wastewater: A critical review. Int. Mater. Rev. 2019, 64, 216–247.
- Doig, L.E.; Liber, K.J.C. An assessment of Hyalella azteca burrowing activity under laboratory sediment toxicity testing conditions. Chemosphere 2010, 81, 261–265.
- Lebrun, M.; Alidou Arzika, I.; Miard, F.; Nandillon, R.; Bayçu, G.; Bourgerie, S.; Morabito, D. Effect of fertilization of a biochar and compost amended technosol: Consequence on Ailanthus altissima growth and As-and Pb-specific root sorption. Soil Use Manag. 2020, 36, 766–772.
- Wang, L.; Ok, Y.S.; Tsang, D.C.; Alessi, D.S.; Rinklebe, J.; Wang, H.; Mašek, O.; Hou, R.; O’Connor, D.; Hou, D. New trends in biochar pyrolysis and modification strategies: Feedstock, pyrolysis conditions, sustainability concerns and implications for soil amendment. Soil Use Manag. 2020, 36, 358–386.
- Leelarungroj, K.; Likitlersuang, S.; Chompoorat, T.; Janjaroen, D. Leaching mechanisms of heavy metals from fly ash stabilised soils. Waste Manag. Res. 2018, 36, 616–623.
- Zha, F.; Ji, C.; Xu, L.; Kang, B.; Yang, C.; Chu, C. Assessment of strength and leaching characteristics of heavy metal–contaminated soils solidified/stabilized by cement/fly ash. Environ. Sci. Pollut. Res. 2019, 53, 30206–30219.
- Liu, L.; Zhu, B.; Wang, G.X. Azoxystrobin-induced excessive reactive oxygen species (ROS) production and inhibition of photosynthesis in the unicellular green algae Chlorella vulgaris. Environ. Sci. Pollut. Res. 2015, 22, 7766–7775.
- Bhat, S.A.; Vig, A.P. Vermistabilization and detoxification of sugar industry sludges by earthworms. In Industrial and Municipal Sludge; Elsevier: Amsterdam, The Netherlands, 2019; pp. 61–81.
- Goel, G.; Kalamdhad, A.S. An investigation on use of paper mill sludge in brick manufacturing. Constr. Build. Mater. 2017, 148, 334–343.
- Idehai, I.M.; Akujieze, C.N. Estimation of landfill gas and its renewable energy potential in Lagos, Nigeria. Int. J. Energy Environ. Eng. 2015, 6, 329–343.
- Wang, G.; Huang, L.; Zhang, Y. Cathodic reduction of hexavalent chromium coupled with electricity generation in microbial fuel cells. Biotechnol. Lett. 2008, 30, 1959–1966.
- Arora, P.K.; Srivastava, A.; Garg, S.K.; Singh, V.P. Recent advances in degradation of chloronitrophenols. Bioresour. Technol. 2018, 250, 902–909.
- Bharagava, R.N.; Saxena, G.; Mulla, S.I. Introduction to industrial wastes containing organic and inorganic pollutants and bioremediation approaches for environmental management. In Bioremediation of Industrial Waste for Environmental Safety; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–18.
- Nascimento, C.W.A.D.; Xing, B. Phytoextraction: A review on enhanced metal availability and plant accumulation. Sci. Agric. 2006, 63, 299–311.
- Lal, B.; Nayak, V.; Sharma, P.; Tedia, K. Effect of combined application of FYM, fly ash and fertilizers on soil properties and paddy grown on degraded land. Curr. World Environ. 2014, 9, 531.
- Tripathi, D.M.; Singh, D.; Tripathi, S. Influence of coal fly-ash on soil properties and productivity of chickpea crop in semi-arid region of Bundelkhand. Curr. World Environ. 2020, 15, 127.
- Schwitzguebel, J. Potential of phytoremediation, an emerging green technology. In Ecosystem Service and Sustainable Watershed Management in North China. Proceedings of International Conference, Beijing, China, 23–25 August 2000; University of Cologne: Köln, Germany, 2000.
- Tahir, U.; Yasmin, A.; Khan, U.H. Phytoremediation: Potential flora for synthetic dyestuff metabolism. J. King Saud Univ.-Sci. 2016, 28, 119–130.
More

