You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Yvaine Wei and Version 1 by Ahmed Elhenawy.
Thiazolidinediones (TZDs) are a kind of DMII drug. The data revealed that compounds (4–6) have higher potency than the reference drugs. Compounds (4–7) were able to regulate hyperlipidemia levels (cholesterol, triglyceride, high-density lipoproteins and low- and very-low-density lipoproteins) to nearly normal value at the 30th day.
- thiazolidinediones
- anti-diabetic
- antioxidant
- molecular docking
1. Introduction
Diabetes mellitus (DM) is a common chronic health problem and around 416 million people worldwide are suffering from it. This figure is expected to reach 618 million cases by 2040 [1,2,3,4][1][2][3][4]. DM was classified into two major classes: “DMI” and “DMII” [5,6][5][6]. More than 90% of cases are diagnosed with DMII (non-insulin-dependent diabetes mellitus; NIDDM). DMII is recognized by tissue resistance to the action of insulin combined with a reduction in insulin secretion because of resistance or deficiency against a beta cell, that requires insulin to control the disease [6]. DMII patients have a two to a fourfold higher risk of cardiovascular disease than non-DM patients [9]. Thiazolidinediones (TZDs) are an efficient DMII drug type [10] (Figure 1). Several TZDs’ derivatives have been marketed for the treatment of DII such as pioglitazone, rosiglitazone, ragaglitazar, balaglitazone [11] (Figure 1). Clinical studies showed that mixed therapy between α-glucosidase inhibitors and PPAR-γ-agonists (peroxisome proliferator-activated receptor [11,12,13]) was a helpful strategy for the treatment of DMII, as acarbose combined with pioglitazone, the first prevents NIDDM and reduces the cardiovascular problem [14]) and the second acting on cardiovascular action and anti-atherosclerosis [15].
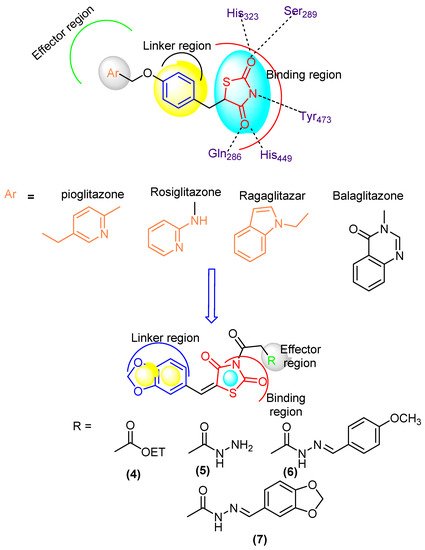

Figure 1.
Designed molecule compliance of Rosiglitazone pharmacophore.
The combination thereby is also effective for older patients with hypertension and DMII [16][7], as well as induced-electrolyte-disturbance in DMII rats [17][8]. Despite the efficiency of the present drugs such as acarbose and TZDs, they can cause adverse effects such as diarrhea, hepatotoxicity, carcinogenesis, oedema, cardiac side effects and obesity [18,19][9][10]. Recently, unimolecular with multi-target drugs have been developed to handle metabolic syndrome, as an inhibitor of Na-dependent-glucose-transporters “SGLTI” [21,22][11][12].
The 2,4-Thiazolidinediones have several potential uses as anti-cancer [23][13], antioxidant [24][14], anti-malarial [25][15], anti-obesity and anti-microbial agents [25,26][15][16].
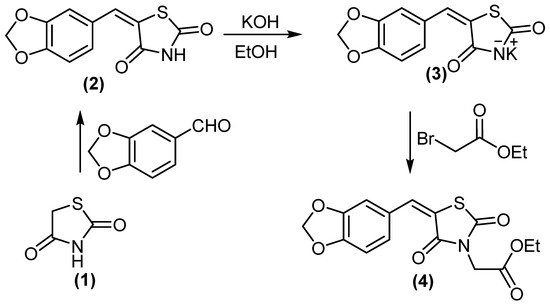
2. Chemistry
5-(benzo[d][1,3]dioxol-5-ylmethylene)thiazolidine-2,4-dione (2) treated with KOH/DMF gave potassium salt derivative (3). Molecule 3 was reacted with ethyl bromoacetate to afford ethyl 2-(5-(benzo[d][1,3]dioxol-5-ylmethylene)-2,4-dioxothiazolidin-3-yl)acetate (4), (Scheme 1). Compound 4 displayed two absorption bands attributed to 2 C=O at 1692 and 1736 cm−1, while its 1H NMR spectrum (DMSO-d6) revealed the signal at δH = 4.18 ppm (2H, CH2).
Scheme 1.
Synthesis of compounds
3
and
4
Compound 4 was hydrazinolyzed to give corresponding hydrazide derivative 5, (Scheme 2). Compound 5 succeeded in condensation with different aldehyde 5 and 6 derivatives, (Scheme 2). Compound 5 showed the absorption bands attributed to amino group at 3251 and 3147 cm−1, the ethoxy resonances disappeared in the NMR proton due to the appearance of the amino group at δ = 7.10 ppm.
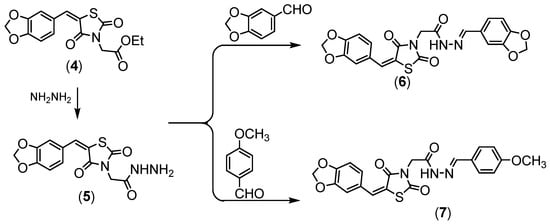
.

Scheme 2.
Synthesis of compounds (
5
–
7
All the newly obtained thiazolidinediones were separated in good yields, with high purity when applied to HPLC analysis.
).
3. Molecular Modeling Studies
3.1. Interaction Stability Based on FMO Analysis
The stability of interactions (inter-and intra-molecular) between kinase and thiazolidinedione based on FMO were examined using DFT/B3YLP/6311G** as implemented in Gaussian 09 package [35][17]. The FMOs included HOMO/LUMO (donating electron/accepting electron) can decide the interaction route between compounds and kinase (Figure 2). The FMOs gap was used to examine the chemical reactivity of the molecule [36][18]. The growing level of HOMO energy reflects the powerful ability for providing electrons with high susceptibility to oxidation, and vice versa [37,38][19][20]. The value for EHOMO was sorted by decreasing order 5 > 7 > 6 > 4 (Table 1). The HOMO sector localized over benzodioxol in all compounds, at the same time the LUMO cloud condensed over the benzodioxol ring.
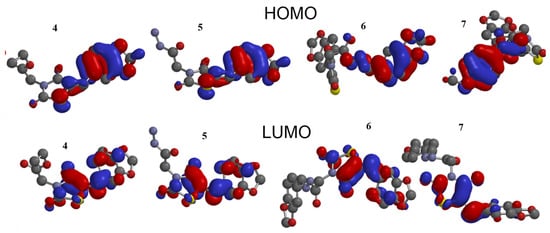

Figure 2.
HOMO and LUMO orbitals density for compounds (
4
–
7
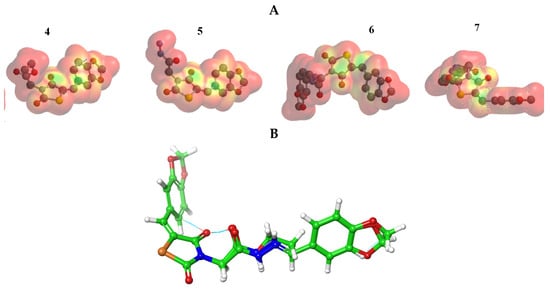
).
2.2. The MEP Analysis
The MEP was mapped to figure out the balance between repulsive interaction (nucleophilic ability) and attractive interaction (electrophilic reactivity) (Figure 3). The orange, yellow and red colors depict the negative power (great electron density area). The colors near to blue shift figured a positive potential, and green color represented intermediate potential ability. MEPs showed that the electron density covered over backbone for compounds (4–7). The expansion of a red region for these compounds is related to electrophilic potency. The variety in shading of MEPs is related to the diversity of electrostatic potential values that can be attributed to expanding electrostatic interactions’ ability and identification between substrate and receptor [43][21].
Figure 3. (A) MEP plot of synthesized compounds (4–7); (B) Overlapping the chemical structures for all compounds (4–7).
4. Biological Study
4.1. The In Vitro α-Amylase Inhibitory Profile
The inhibitory potential was examined for compounds (4–7) against the α-amylase enzyme. The concentrations were plotted against inhibition to determine IC50 (concentration required to inhibit 50% of enzyme) using the non-linear regression method (Figure 54). Compounds (4–6) showed higher inhibitory potential (IC50 = 11.8 to 21.34 µg/mL) than “STD” standard acarbose (IC50 =24.1 µg/mL). Meanwhile 7 with IC50 = (57.34 µg/mL) showed lower potency than acarbose with significant (p < 0.05) when compared to positive control. The parent compound which bore the ester (4), hydrazide (5) and benzodioxole (6) corners had a more promising activity than acarbose (Figure 5). On the other hand, the inhibition potency decreased by the introduction of the methoxyphenyl fragment to the hydrazone in the molecular skeleton, as in 7. This efficiency can be attributed to increasing hydrophilicity, which can interact with the hydrophobic part of the active site.
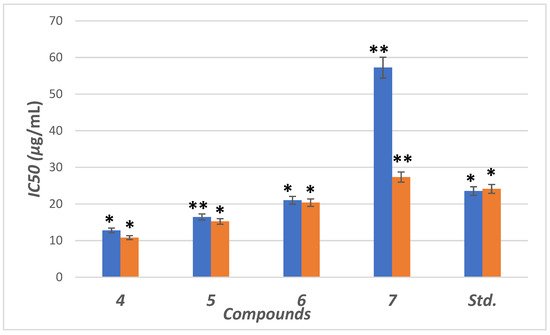

Figure 54. IC50 in (μg/mL) for 4–7 compared to ascorbic and acarbose, respectively, as standard drugs against DPPH radical (blue column) and α-amylase enzyme (orange column), respectively. Data analyzed by one-way ANOVA followed by LSD test and expressed as mean ± SEM from five observations; stars represent change as compared to control; ** indicates p < 0.01 and * shows p < 0.05.
4.2. The In Vitro Antioxidant Activity
The antioxidant compounds can protect β-cells from reactive oxygen species (ROS) and, therefore, can prevent diabetes-induced by ROS [51][22]. In addition, ROS induces oxidative stress that leads to lipid peroxidation, protein glycation/oxidation and nitration, enzyme inactivation and DNA damage, which leads to the development of various pathological conditions such as diabetes mellitus (DM) and neurodegenerative diseases, which in turn can be neutralized by the presence of endogenous or exogenous antioxidant systems [52,53][23][24].
4.3. Lipid Profile
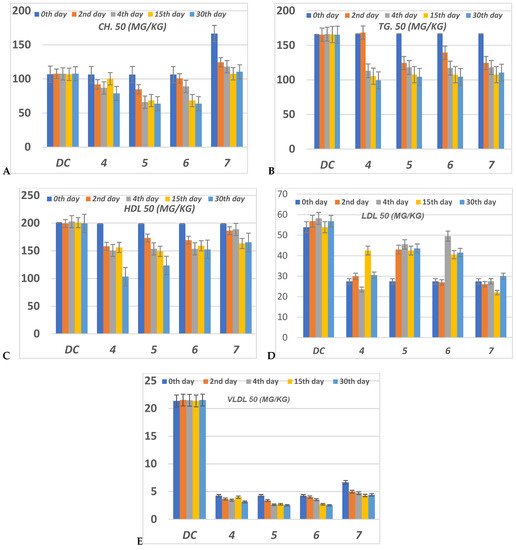
The serious side effect related to diabetes is hyperlipidemia [54,55][25][26]. The insulin deficiency promotes adipocytes to generate fatty acids, hepatic phospholipids and cholesterol [56,57][27][28]. Thus, the levels of cholesterol (CH), triglyceride (TG), high (HDL), low (LDL) and very low (VLDL) density lipoproteins, in serum for alloxan-loaded diabetes have been measured at 50 mg/Kg concentration (Figure 75). All lipid parameters for all tested compounds have (p < 0.05) compared to the diabetic control group (Figure 75).
Figure 75. The level of (A) CH (Cholesterol); (B) TG (Triglyceride); (C) HDL (High-density lipoprotein); (D) LDL (Low-density lipoprotein) and (E) VLDL (Very low-density lipoprotein) in serum of hyperglycemic loaded rats. 50 (mg/Kg) at 0th day (pre-drug values), 2nd and 4th days (post-drug values) for each group of compounds 4–7 and compared to diabetic control (DC). Data were analyzed by one-way ANOVA followed by LSD test and expressed as mean ± SEM from five observations.
5. Conclusions
An antihyperglycemic compound, based on the thiazolidinedione scaffold, was synthesized and characterized. The FMOs and global reactivity data indicated that the intramolecular charge transfer takes place between the thiazolidine-2,4-dione and benzodioxole corner in two directions. The MEP for these compounds represented that they have promising electrophilicity, that may enhance the attacking chance over a polar active site of the receptor. The molecular docking into the PPAR-γ and α-amylase showed a high binding affinity for the synthesized compounds and maybe a suitable regulator for both kinases. The in vitro inhibition potency via the α-amylase and scavenging radical was evaluated and represented that the parent compound bearing these corners ester 4, hydrazide 5 and benzodioxole 6 are more efficient than reference drugs. This efficacy decreased in the presence of methoxyphenyl group 7 in the molecular skeleton. The anti-diabetic and anti-hyperlipidemic activities in vitro showed that all of the used concentrations for compounds 4–7 didn’t show an obvious reduction in the BGL during pre-and post-treatment compared to DC, while after the 30th days of treatment, they decreased the BGL at all concentrations. The ester 5 hybrid was the most potent regarding the regulation of BGL at different concentrations on the 30th day. All compounds didn’t exhibit any mortality at 50 mg/Kg concentration during the whole experiment. Moreover, all compounds enhanced hyperlipidemic levels nearly to the normal level.
References
- Zimmet, P.; Alberti, K.G.; Shaw, J. Global and societal implications of the diabetes epidemic. Nature 2001, 414, 782–787.
- Wild, S.H.; Roglic, G.; Green, A.; Sicree, R.; King, H. Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030: Response to Rathman and Giani. Diabetes Care 2004, 27, 2569.
- Bhutani, R.; Pathak, D.P.; Kapoor, G.; Husain, A.; Kant, R.; Iqbal, M.A. Synthesis, Molecular modelling studies and ADME prediction of benzothiazole clubbed oxadiazole-Mannich bases, and evaluation of their Anti-diabetic activity through in-vivo model. Bioorganic Chem. 2018, 77, 6–15.
- Cho, N.H.; Shaw, J.E.; Karuranga, S.; Huang, Y.; da Rocha Fernandes, J.D.; Ohlrogge, A.W.; Malanda, B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281.
- Diamond, J. The double puzzle of diabetes. Nature 2003, 423, 599–602.
- King, H.; Aubert, R.E.; Herman, W.H. Global burden of diabetes, 1995–2025: Prevalence, numerical estimates, and projections. Diabetes Care 1998, 21, 1414–1431.
- Del Prato, S.; Chilton, R. Practical strategies for improving outcomes in T2DM: The potential role of pioglitazone and DPP4 inhibitors. Diabetes Obes. Metab. 2018, 20, 786–799.
- Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001, 414, 813–820.
- Nanjan, M.J.; Mohammed, M.; Kumar, B.P.; Chandrasekar, M.J. Thiazolidinediones as antidiabetic agents: A critical review. Bioorganic Chem. 2018, 77, 548–567.
- Brownlee, M.; Hirsch, I.B. Glycemic variability: A hemoglobin A1c–independent risk factor for diabetic complications. JAMA 2006, 295, 1707–1708.
- Chiasson, J.L.; Josse, R.G.; Gomis, R.F.; Hanefeld, M.; Karasik, A.; Laakso, M. Acarbose for the prevention of Type 2 diabetes, hypertension and cardiovascular disease in subjects with impaired glucose tolerance: Facts and interpretations concerning the critical analysis of the STOP-NIDDM Trial data. Diabetologia 2004, 47, 969–975.
- Yokoyama, H.; Katakami, N.; Yamasaki, Y. Recent advances of intervention to inhibit progression of carotid intima-media thickness in patients with type 2 diabetes mellitus. Stroke 2006, 37, 2420–2427.
- Brownlee, M. The pathobiology of diabetic complications: A unifying mechanism. Diabetes 2005, 54, 1615–1625.
- Xiao, B.; Xiao, Y.; Ning, H.; Han, X.; Li, W.; Ma, Y.; Zhao, N.; Du, G.; Dong, Y.; Jung, J.H. In vitro dual-target activities and in vivo antidiabetic effect of 3-hydroxy-N-(p-hydroxy-phenethyl) phthalimide in high-fat diet and streptozotocin-induced diabetic golden hamsters. Med. Chem. Res. 2020, 29, 2077–2088.
- Khatoon, H.; Najam, R. Effects of Rosiglitazone and Acarbose (with and without Cornstarch Diet) on serum electrolytes in diabetic rats. J. Appl. Pharm. Sci. 2012, 2, 50.
- Bennett, W.L.; Maruthur, N.M.; Singh, S.; Segal, J.B.; Wilson, L.M.; Chatterjee, R.; Marinopoulos, S.S.; Puhan, M.A.; Ranasinghe, P.; Block, L. Comparative effectiveness and safety of medications for type 2 diabetes: An update including new drugs and 2-drug combinations. Ann. Intern. Med. 2011, 154, 602–613.
- Sims, H.; Smith, K.H.; Bramlage, P.; Minguet, J. Sotagliflozin: A dual sodium-glucose co-transporter-1 and-2 inhibitor for the management of Type 1 and Type 2 diabetes mellitus. Diabet. Med. 2018, 35, 1037–1048.
- Garcia-Vallvé, S.; Guasch, L.; Tomas-Hernández, S.; del Bas, J.M.; Ollendorff, V.; Arola, L.; Pujadas, G.; Mulero, M. Peroxisome Proliferator-Activated Receptor γ (PPARγ) and Ligand Choreography: Newcomers Take the Stage: Miniper-spective. J. Med. Chem. 2015, 58, 5381–5394.
- May, L.D.; Lefkowitch, J.H.; Kram, M.T.; Rubin, D.E. Mixed hepatocellular–cholestatic liver injury after pioglitazone therapy. Ann. Intern. Med. 2002, 136, 449–452.
- Okumura, T. Mechanisms by which thiazolidinediones induce anti-cancer effects in cancers in digestive organs. J. Gastroenterol. 2010, 45, 1097–1102.
- Devji, T.; Reddy, C.; Woo, C.; Awale, S.; Kadota, S.; Carrico-Moniz, D. Pancreatic anticancer activity of a novel geranylgeranylated coumarin derivative. Bioorganic Med. Chem. Lett. 2011, 21, 5770–5773.
- Naim, M.J.; Alam, M.J.; Ahmad, S.; Nawaz, F.; Shrivastava, N.; Sahu, M.; Alam, O. Therapeutic journey of 2, 4-thiazolidinediones as a versatile scaffold: An insight into structure activity relationship. Eur. J. Med. Chem. 2017, 129, 218–250.
- Bahare, R.S.; Ganguly, S.; Agrawal, R.; Dikshit, S.N. Thiazolidine: A Potent Candidate for Central Nervous System Diseases. Cent. Nerv. Syst. Agents Med. Chem. (Former. Curr. Med. Chem.-Cent. Nerv. Syst. Agents) 2017, 17, 26–29.
- Frisch, M.; Trucks, G.; Schlegel, H.; Scuseria, G.; Robb, M.; Cheeseman, J.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G. Gaussian 09, Revision D. 01; Gaussian, Inc.: Wallingford, CT, USA, 2013.
- Luque, F.J.; López, J.M.; Orozco, M. Perspective on “Electrostatic interactions of a solute with a continuum. A direct utilization of ab initio molecular potentials for the prevision of solvent effects”. In Theoretical Chemistry Accounts; Springer: Berlin/Heidelberg, Germany, 2000; pp. 343–345.
- Sundriyal, S.; Viswanad, B.; Ramarao, P.; Chakraborti, A.K.; Bharatam, P.V. New PPARγ ligands based on barbituric acid: Virtual screening, synthesis and receptor binding studies. Bioorganic Med. Chem. Lett. 2008, 18, 4959–4962.
- Siddiqui, S.A.; Rasheed, T.; Faisal, M.; Pandey, A.K.; Khan, S.B. Electronic structure, nonlinear optical properties, and vibrational analysis of gemifloxacin by density functional theory. J. Spectrosc. 2012, 27, 185–206.
- Ha, N.V.; Dat, D.T.; Nguyet, T.T. Stereoelectronic Properties of 1,2,4-Triazole-Derived N-heterocyclic Carbenes—A Theoretical Study. VNU J. Sci. Nat. Sci. Technol. 2019, 35, 55–62.
- Alminderej, F.; Bakari, S.; Almundarij, T.I.; Snoussi, M.; Aouadi, K.; Kadri, A. Antioxidant Activities of a New Chemotype of Piper cubeba L. Fruit Essential Oil (Methyleugenol/Eugenol): In Silico Molecular Docking and ADMET Studies. Plants 2020, 9, 1534.
- Abrahamse, H.; George, S. Redox Potential of Antioxidants in Cancer Progression and Prevention. Antioxidants 2020, 9, 1156.
- Famuyiwa, S.O.; Sanusi, K.; Faloye, K.O.; Yilmaz, Y.; Ceylan, Ü. Antidiabetic and antioxidant activities: Is there any link between them? New J. Chem. 2019, 43, 13326–13329.
- Gandhi, G.R.; Sasikumar, P. Antidiabetic effect of Merremia emarginata Burm. F. in streptozotocin induced diabetic rats. Asian Pac. J. Trop. Biomed. 2012, 2, 281–286.
- Pareek, H.; Sharma, S.; Khajja, B.S.; Jain, K.; Jain, G. Evaluation of hypoglycemic and anti-hyperglycemic potential of Tridax procumbens (Linn.). BMC Complementary Altern. Med. 2009, 9, 48.
- Gadadare, R.; Mandpe, L.; Pokharkar, V. Ultra rapidly dissolving repaglinide nanosized crystals prepared via bottom-up and top-down approach: Influence of food on pharmacokinetics behavior. AAPS PharmSciTech 2015, 16, 787–799.
- Sharma, M.; Kohli, S.; Dinda, A. In-vitro and in-vivo evaluation of repaglinide loaded floating microspheres prepared from different viscosity grades of HPMC polymer. Saudi Pharm. J. 2015, 23, 675–682.
More
