Seeds are the reproductive units of higher plants. They have a significant place in agriculture and plant diversity maintenance. Because they are dehydrated, they can remain viable in the environment for centuries. The dry seed is a metabolically inactive organism, but well organized to protect its components and enter intensive repair to restore metabolic activities upon imbibition for the completion of germination.
- seed
- hydration force
- energy
- germination
- dormancy
- ageing
- PM H+ ATPase
1. Dry Seed: Well-Organized to Resist
1.1. The Seed, a Special New Individual
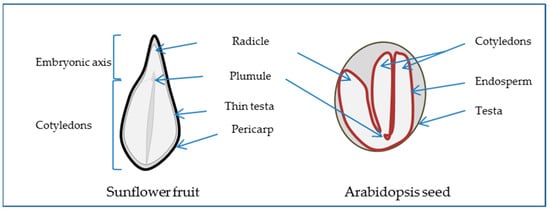
12.2. Water, “Matrix of Life”
If water is the matrix of life [5], dry seeds can hardly be considered as alive and yet they bear life in the form of the embryo. Water is an essential participant in the chemistry of life by sustaining the biochemistry of the cell. It acts as a liquid and solvent for biochemical reactions, but also influences macromolecule structures [6]. Water participates in the catalytic function of proteins and nucleic acids and physically in hydrophobic associated protein folding and complex formation through the hydrogen bond [7]. It was shown that in desiccated Arabidopsis seeds the chromatin is highly condensed and can be de-condensated after hydration [8]. The property of the seed to undergo a reversible chromatin condensation/de-condensation enables to withstand desiccation and the entry in active metabolism during imbibition.
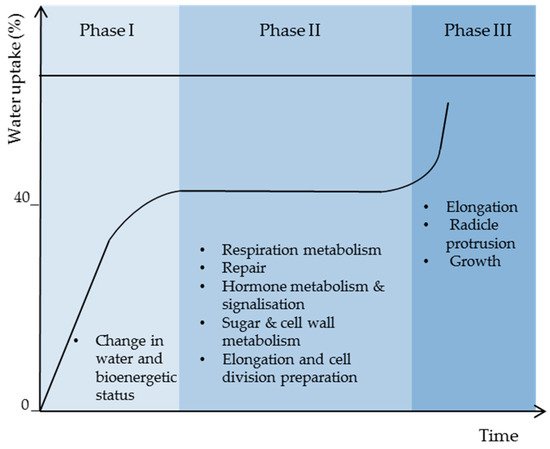
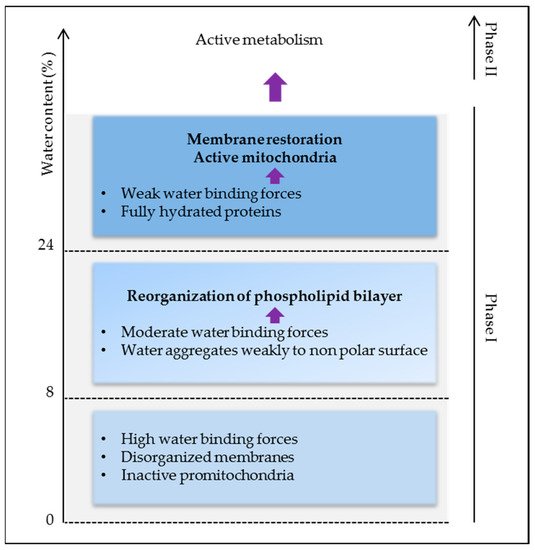
Thus, high water binding forces in dry seeds are responsible for the lack of stability and activity of biomolecules causing low metabolism and energy. As membrane reorganization is one of the first events in the initiation of cell energization, more water (>24%) is needed to activate protein reorganization and activity for full plasma membrane and mitochondrial energy restoration (Figure 3).
12.3. Respiration Resumption
12.4. Plasma Membrane Potential
23. Seed Dormancy: Higher Level of Resistance
23.1. Seed Metabolism and Dormancy
After-ripening has fascinated researchers because dormancy is alleviated in dry conditions, suggesting that some processes operate in the dry seed. Biological reactions have been investigated and transcriptional programs have been proposed to be involved in the regulation of after-ripening-mediated seed dormancy alleviation in several seeds [34][35][36][37][38]. Given the restricted molecular mobility due to the glassy state in dry seed cells, the existence of a hydrated pocket within the cell enabling gene transcription has been hypothesized [34]. To address this issue, Meimoun et al. [3] investigated transcriptomic changes after the after-ripening period in sunflower seeds using two protocols, one allowing dormancy alleviation but not the other, in order to differentiate between changes in gene expression associated with dormancy alleviation and those associated with storage only. They showed that there is no significant variation between conditions, suggesting that gene expression did not take place during after-ripening, in agreement with the absence of metabolic activity in dry seeds [3].
Non-enzymatic oxidations are possible in low hydrated seeds and represent the most plausible lead to explain the observed molecular changes reported during after-ripening [39]. Indeed, mRNA oxidation was shown to be associated with dormancy release during after-ripening in sunflower and wheat [40][41], which alters the stability of stored mRNAs, being finally degraded or translated into non-functional proteins [42]. However, if a fraction of stored mRNA is inactivated, the one involved in germination has to be protected from oxidation.
23.2. Internal Determinants of Dormancy
It is well established that dormancy is regulated by the hormonal balance between the main positive regulator abscisic acid (ABA) and negative ones, such as hormones like gibberellic acid (GA), ethylene (ET), auxins, or brassinosteroids, as well as some other molecules, like ROS or nitric oxide (NO). The involvement of each of them and their interactions in the whole process of germination depend on the structure of the seed and the environment. Nevertheless, ABA represents the highly conserved component of the process across species and the unique dormancy determinant as opposed to the multiple stimulants of germination. Thus, to illustrate the regulation and function of hormones in the physiology of germination, without elaborating on all the hormones and their complex signaling, the case of the ABA is the most appropriate. Mitochondria play a central role in energy supply and they are also associated with ABA sensitivity based on works showing that several mutants of RNA processing for subunits of the electron chain display reduced ABA sensitivity. This regulation involves retrograde, anterograde, and inter-organelle signals in the transcription control of the ABA biosynthesis gene, NCED [43]. On the other hand, Paszkiewicz et al. [20] have shown that mitochondrial dynamics associated with germination condition was slightly affected by ABA treatment, arguing that mitochondrion reactivation depends only on the physical conditions of hydration and temperature. Based on these works, the optimal differentiation and functioning of mitochondrion are associated with an ABA sensitivity decrease. Accordingly, it is easy to consider that in dormant seeds, the impairment of mitochondrial activity occurs. However, it has long been established that inhibitors of oxidative phosphorylation such as cyanid can break dormancy. This paradox has still not been elucidated. The activation of the pentose phosphate pathway, the metabolic pathway that supplies reducing energy to cells, has been the most plausible hypothesis proposed [44]. Indeed, in reduced mitochondrial activity, glycolysis is activated to obtain ATP, a phenomenon known the “Pasteur effect”, leading to pyruvate production and the accumulation of fermentation by-products. Thus, anaerobic metabolism facilitates reserve breakdown and it might operate, in normal conditions, at the onset of germination when the mitochondria are not yet fully reactivated. All these data point to the importance of cell metabolism and energy regulation for successful germination. On the other hand, it was proposed that the ABA inhibition of growth in germinating Arabidopsis seeds is driven by its inhibitory action on PM H+-ATPase activity [45]. ABA inhibition was less effective in Arabidopsis mutants with increased capacity for H+ efflux, suggesting that cytosolic acidification due to reduced H+-ATPase activity was the main mechanism driving growth inhibition [45]. Similarly, in sunflower, ABA induced the inhibition of PM H+-ATPase in non-dormant seeds, which display hyperpolarization and subsequent membrane energization. Meanwhile, in dormant seeds, PM H+-ATPase activity was reduced even if the corresponding proteins were present and the levels of ATP were comparable to that on ND [33]. PM H+-ATPase activity is also regulated by ROS and ethylene in the opposite way [33], as proposed in the model presented Figure 4.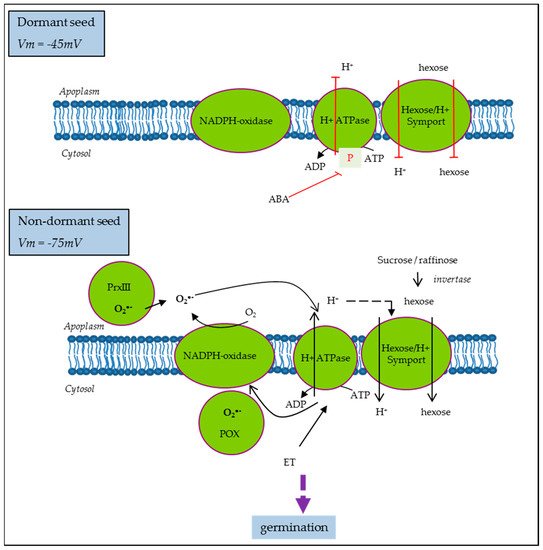
23.3. Environmental Impact on Dormancy
Environmental factors are of high importance in the awakening of the seed. Their effect on seed performance was shown to surpass genetic impact [47]. Temperature and soil moisture oscillations are the major players under natural conditions. Indeed, alternating temperatures more than constant ones can promote germination via the interplay between ROS signaling and hormones [48]. On the other hand, light and nitrate also play important roles. Their effects are associated in dormancy cycling [49][50][51]. Seed sensitivity to both of them depends on the season and depth of dormancy. A low concentration of nitrate (around 0.1 mM) is able to promote seed germination in several species [49]. Several evidences converge towards nitrate induction of CYP707A2 leading to ABA decrease more than GA biosynthesis in dormancy breakdown [49]. However, GA biosynthesis gene involvement has also been reported in response to environmental cues in Arabidopsis seeds from lab but also soil seed bank experiences [52][53]. Moreover, the analysis of the whole transcriptome change by nitrate treatment during seed imbibition showed the upregulation of genes involved in nitrate assimilation and transport, hormone metabolism, and energy, such as Glucose-6-phosphate dehydrogenase2, highlighting the importance of the pentose phosphate pathway [54]. In fact, dormancy is tightly regulated in natural conditions as in the soil, when seeds experience several scenarios of temperature, light, nitrate, and moisture, as well as microbial environment. The latter corresponds to a wide range of microbes, as pathogenic ones can induce decrease in seed longevity due to infection, and others can influence seed dormancy by breaking down the seed coat [55][56].
34. Seeds: The Ability to Recover from Ageing
34.1. Seed Ageing
Seed ageing was defined as the loss of seed quality and viability over time [57]. Aged seeds germinate poorly giving abnormal seedlings or ultimately are unable to germinate. Orthodox seeds are resistant to ageing for very long time because they have a very low water content, resulting in reduced cell metabolism especially respiration which is responsible for the major production of ROS [58]. Indeed, as for the dry after-ripening process described above, enzymatic reactions and respiration are restricted by the lack of free available water preventing cellular damage. It was shown that long term storage resulting in seed loss of viability is associated with the impairment of mitochondrial activity and protein synthesis machinery [59][60]. Mitochondrion membrane integrity was identified as the primary target for ageing leading to the deregulation of its oxidative properties [61]. ROS are considered as the major cause of seed deterioration due to the oxidation of its components [62].
34.2. Seed Priming
Seeds possess effective repair machinery to cope with ageing-associated oxidative damage. Several non-enzymatic antioxidants have been proposed to be determinant in seed longevity, e.g., glutathione or ascorbic acid [63][64]. The antioxidant enzymes are also of importance in ROS detoxification, such as catalase, superoxide dismutase, ascorbate peroxidase, or glutathione reductase [65][66]. Other enzymes acting on specific macromolecules are also activated, such as DNA or protein repair enzymes [67][68][69]. Such machinery operates when seed hydration occurred, and its efficiency depends on the plant species and the extent of ageing damage. Based on this feature, a priming technique has been developed to improve seed quality from alterations caused by several stresses. Priming treatment consists of seed pre-hydration with a controlled amount of water which does not allow radicle elongation, i.e., a water amount corresponding phase II of the germination sensu stricto, which is sufficient to trigger the reparation processes. The addition of beneficial molecules, such as antioxidants or hormones, during priming treatment can further increase seed reparation and subsequent quality. Several priming techniques have been developed depending on the plant species and subsequent use. They all lead to an improvement of seed performance under variable environmental conditions [70]. It was shown that repair mechanisms and oxidative management in primed seeds represent the main processes associated with priming induced germination improvement, but DNA replication, cell cycle advancement, the modification of the membrane structure, and restoration of mitochondrial integrity were also proposed to explain the priming effect in germination improvement [71]. In fact, the seed engages growth preparation processes during imbibition while maintaining the desiccation tolerance machinery which allowed a successful dehydration after the priming treatment. The dried primed seed is ready to grow better, even under stress conditions.
45. Conclusion
Understanding the successful entry and exit from desiccation is fundamental for the improvement of seed germination in the coming challenging conditions due to global warming. The application in plant germplasm conservation in seed banks is of high importance in the maintenance of genetic resources for food and environment security. In this review, several layers of regulations of seed performance were shown, from the organization and physical protection of cell components to the regulation of several signaling processes in a coordinated crosstalk. However, their implementation and coordination of these mechanisms during seed development deserve more investigations.
References
- Belmonte, M.F.; Kirkbride, R.C.; Stone, S.L.; Pelletier, J.M.; Bui, A.Q.; Yeung, E.C.; Hashimoto, M.; Fei, J.; Harada, C.M.; Munoz, M.D.; et al. Comprehensive Developmental Profiles of Gene Activity in Regions and Subregions of the Arabidopsis Seed. Proc. Natl. Acad. Sci. USA 2013, 110, E435–E444.
- Ingram, G.C. Family Life at Close Quarters: Communication and Constraint in Angiosperm Seed Development. Protoplasma 2010, 247, 195–214.
- Meimoun, P.; Mordret, E.; Langlade, N.B.; Balzergue, S.; Arribat, S.; Bailly, C.; El-Maarouf-Bouteau, H. Is Gene Transcription Involved in Seed Dry After-Ripening? PLoS ONE 2014, 9, e86442.
- Ballesteros, D.; Walters, C. Detailed Characterization of Mechanical Properties and Molecular Mobility within Dry Seed Glasses: Relevance to the Physiology of Dry Biological Systems: Molecular Mobility within the Glass of Dry Seed. Plant J. 2011, 68, 607–619.
- Szent-Györgyi, A. Cell-Associated Water; Academic Press: New York, NY, USA, 1979; pp. 363–413.
- Ball, P. Water as an Active Constituent in Cell Biology. Chem. Rev. 2008, 108, 74–108.
- Bellissent-Funel, M.-C.; Hassanali, A.; Havenith, M.; Henchman, R.; Pohl, P.; Sterpone, F.; van der Spoel, D.; Xu, Y.; Garcia, A.E. Water Determines the Structure and Dynamics of Proteins. Chem. Rev. 2016, 116, 7673–7697.
- van Zanten, M.; Koini, M.A.; Geyer, R.; Liu, Y.; Brambilla, V.; Bartels, D.; Koornneef, M.; Fransz, P.; Soppe, W.J.J. Seed Maturation in Arabidopsis Thaliana Is Characterized by Nuclear Size Reduction and Increased Chromatin Condensation. Proc. Natl. Acad. Sci. USA 2011, 108, 20219–20224.
- Angelovici, R.; Galili, G.; Fernie, A.R.; Fait, A. Seed Desiccation: A Bridge between Maturation and Germination. Trends Plant Sci. 2010, 15, 211–218.
- Borisjuk, L.; Rolletschek, H. The Oxygen Status of the Developing Seed: Tansley Review. New Phytol. 2009, 182, 17–30.
- Logan, D.C.; Millar, A.H.; Sweetlove, L.J.; Hill, S.A.; Leaver, C.J. Mitochondrial Biogenesis during Germination in Maize Embryos. Plant Physiol. 2001, 125, 662–672.
- Howell, K.A.; Millar, A.H.; Whelan, J. Ordered Assembly of Mitochondria During Rice Germination Begins with Promitochondrial Structures Rich in Components of the Protein Import Apparatus. Plant Mol. Biol. 2006, 60, 201–223.
- Nawa, Y.; Asahi, T. Rapid Development of Mitochondria in Pea Cotyledons during the Early Stage of Germination. Plant Physiol. 1971, 48, 671–674.
- Morohashi, Y.; Bewley, J.D. Development of Mitochondrial Activities in Pea Cotyledons: Influence of desiccation during and following germination of the axis. Plant Physiol. 1980, 66, 637–640.
- Morohashi, Y.; Bewley, J.D.; Yeung, E.C. Biogenesis of Mitochondria in Imbibed Peanut Cotyledons: II. Development of light and heavy mitochondria. Plant Physiol. 1981, 68, 318–323.
- Ehrenshaft, M.; Brambl, R. Respiration and Mitochondrial Biogenesis in Germinating Embryos of Maize. Plant Physiol. 1990, 93, 295–304.
- Law, S.R.; Narsai, R.; Whelan, J. Mitochondrial Biogenesis in Plants during Seed Germination. Mitochondrion 2014, 19, 214–221.
- Czarna, M.; Kolodziejczak, M.; Janska, H. Mitochondrial Proteome Studies in Seeds during Germination. Proteomes 2016, 4, 19.
- Benamar, A.; Rolletschek, H.; Borisjuk, L.; Avelange-Macherel, M.-H.; Curien, G.; Mostefai, H.A.; Andriantsitohaina, R.; Macherel, D. Nitrite–Nitric Oxide Control of Mitochondrial Respiration at the Frontier of Anoxia. Biochim. Biophys. Acta BBA—Bioenerg. 2008, 1777, 1268–1275.
- Paszkiewicz, G.; Gualberto, J.M.; Benamar, A.; Macherel, D.; Logan, D.C. Arabidopsis Seed Mitochondria Are Bioenergetically Active Immediately upon Imbibition and Specialize via Biogenesis in Preparation for Autotrophic Growth. Plant Cell 2017, 29, 109–128.
- Attucci, S.; Carde, J.P.; Raymond, P.; Saint-Gès, V.; Spiteri, A.; Pradet, A. Oxidative Phosphorylation by Mitochondria Extracted from Dry Sunflower Seeds. Plant Physiol. 1991, 95, 390–398.
- Nietzel, T.; Mostertz, J.; Ruberti, C.; Née, G.; Fuchs, P.; Wagner, S.; Moseler, A.; Müller-Schüssele, S.J.; Benamar, A.; Poschet, G.; et al. Redox-Mediated Kick-Start of Mitochondrial Energy Metabolism Drives Resource-Efficient Seed Germination. Proc. Natl. Acad. Sci. USA 2020, 117, 741–751.
- Vertucci, C.W.; Leopold, A.C. Bound Water in Soybean Seed and Its Relation to Respiration and Imbibitional Damage. Plant Physiol. 1984, 75, 114–117.
- Simon, E.W. Phospholipids and plant membrane permeability. New Phytol. 1974, 73, 377–420.
- Yu, X.; Li, A.; Li, W. How Membranes Organize during Seed Germination: Three Patterns of Dynamic Lipid Remodelling Define Chilling Resistance and Affect Plastid Biogenesis: Remodelling of Membrane Lipids during Germination. Plant Cell Environ. 2015, 38, 1391–1403.
- Lin, Y.; Xin, X.; Yin, G.; He, J.; Zhou, Y.; Chen, J.; Lu, X. Membrane Phospholipids Remodeling upon Imbibition in Brassica Napus L. Seeds. Biochem. Biophys. Res. Commun. 2019, 515, 289–295.
- Sze, H.; Li, X.; Palmgren, M.G. Energization of Plant Cell Membranes by H+-Pumping ATPases: Regulation and Biosynthesis. Plant Cell 1999, 11, 677–689.
- Haruta, M.; Burch, H.L.; Nelson, R.B.; Barrett-Wilt, G.; Kline, K.G.; Mohsin, S.B.; Young, J.C.; Otegui, M.S.; Sussman, M.R. Molecular Characterization of Mutant Arabidopsis Plants with Reduced Plasma Membrane Proton Pump Activity. J. Biol. Chem. 2010, 285, 17918–17929.
- Falhof, J.; Pedersen, J.T.; Fuglsang, A.T.; Palmgren, M. Plasma Membrane H+-ATPase Regulation in the Center of Plant Physiology. Mol. Plant 2016, 9, 323–337.
- Lang, V.; Pertl-Obermeyer, H.; Safiarian, M.J.; Obermeyer, G. Pump up the Volume—A Central Role for the Plasma Membrane H+ Pump in Pollen Germination and Tube Growth. Protoplasma 2014, 251, 477–488.
- Pedersen, J.T.; Falhof, J.; Ekberg, K.; Buch-Pedersen, M.J.; Palmgren, M. Metal Fluoride Inhibition of a P-Type H+ Pump: Stabilization of the phosphoenzyme intermediate contributes to post-translational pump activation. J. Biol. Chem. 2015, 290, 20396–20406.
- Pedersen, J.T.; Kanashova, T.; Dittmar, G.; Palmgren, M. Isolation of Native Plasma Membrane H+-ATP Ase (Pma1p) in Both the Active and Basal Activation States. FEBS Open Bio 2018, 8, 774–783.
- De Bont, L.; Naim, E.; Arbelet-Bonnin, D.; Xia, Q.; Palm, E.; Meimoun, P.; Mancuso, S.; El-Maarouf-Bouteau, H.; Bouteau, F. Activation of Plasma Membrane H+-ATPases Participates in Dormancy Alleviation in Sunflower Seeds. Plant Sci. 2019, 280, 408–415.
- Leubner-Metzger, G. Beta-1,3-Glucanase Gene Expression in Low-Hydrated Seeds as a Mechanism for Dormancy Release during Tobacco after-Ripening. Plant J. Cell Mol. Biol. 2005, 41, 133–145.
- Bove, J.; Lucas, P.; Godin, B.; Ogé, L.; Jullien, M.; Grappin, P. Gene Expression Analysis by CDNA-AFLP Highlights a Set of New Signaling Networks and Translational Control during Seed Dormancy Breaking in Nicotiana Plumbaginifolia. Plant Mol. Biol. 2005, 57, 593–612.
- Leymarie, J.; Bruneaux, E.; Gibot-Leclerc, S.; Corbineau, F. Identification of Transcripts Potentially Involved in Barley Seed Germination and Dormancy Using CDNA-AFLP. J. Exp. Bot. 2006, 58, 425–437.
- Gao, F.; Jordan, M.C.; Ayele, B.T. Transcriptional Programs Regulating Seed Dormancy and Its Release by After-Ripening in Common Wheat (Triticum Aestivum L.). Plant Biotechnol. J. 2012, 10, 465–476.
- Liu, A.; Gao, F.; Kanno, Y.; Jordan, M.C.; Kamiya, Y.; Seo, M.; Ayele, B.T. Regulation of Wheat Seed Dormancy by After-Ripening Is Mediated by Specific Transcriptional Switches That Induce Changes in Seed Hormone Metabolism and Signaling. PLoS ONE 2013, 8, e56570.
- El-Maarouf-Bouteau, H.; Bailly, C. Oxidative Signaling in Seed Germination and Dormancy. Plant Signal. Behav. 2008, 3, 175–182.
- Bazin, J.; Langlade, N.; Vincourt, P.; Arribat, S.; Balzergue, S.; El-Maarouf-Bouteau, H.; Bailly, C. Targeted MRNA Oxidation Regulates Sunflower Seed Dormancy Alleviation during Dry After-Ripening. Plant Cell 2011, 23, 2196–2208.
- Gao, F.; Rampitsch, C.; Chitnis, V.R.; Humphreys, G.D.; Jordan, M.C.; Ayele, B.T. Integrated Analysis of Seed Proteome and MRNA Oxidation Reveals Distinct Post-Transcriptional Features Regulating Dormancy in Wheat (Triticum Aestivum L.). Plant Biotechnol. J. 2013, 11, 921–932.
- Katsuya-Gaviria, K.; Caro, E.; Carrillo-Barral, N.; Iglesias-Fernández, R. Reactive Oxygen Species (ROS) and Nucleic Acid Modifications during Seed Dormancy. Plants 2020, 9, 679.
- Nonogaki, H. The Long-Standing Paradox of Seed Dormancy Unfolded? Trends Plant Sci. 2019, 24, 989–998.
- Bewley, J.D.; Black, M. Seeds; Springer: Boston, MA, USA, 1994; pp. 1–33. Available online: https://link.springer.com/chapter/10.1007/978-1-4899-1002-8_1 (accessed on 15 October 2021).
- Planes, M.D.; Niñoles, R.; Rubio, L.; Bissoli, G.; Bueso, E.; García-Sánchez, M.J.; Alejandro, S.; Gonzalez-Guzmán, M.; Hedrich, R.; Rodriguez, P.L.; et al. A Mechanism of Growth Inhibition by Abscisic Acid in Germinating Seeds of Arabidopsis thaliana Based on Inhibition of Plasma Membrane H+-ATPase and Decreased Cytosolic PH, K+, and Anions. J. Exp. Bot. 2015, 66, 813–825.
- Penfield, S. Seed Dormancy and Germination. Curr. Biol. 2017, 27, R874–R878.
- de Souza Vidigal, D.; He, H.; Hilhorst, H.W.M.; Willems, L.A.J.; Bentsink, L. Arabidopsis in the Wild—The Effect of Seasons on Seed Performance. Plants 2020, 9, 576.
- Matilla, A.J. Seed Dormancy: Molecular Control of Its Induction and Alleviation. Plants 2020, 9, 1402.
- Duermeyer, L.; Khodapanahi, E.; Yan, D.; Krapp, A.; Rothstein, S.J.; Nambara, E. Regulation of Seed Dormancy and Germination by Nitrate. Seed Sci. Res. 2018, 28, 150–157.
- Yan, A.; Chen, Z. The Control of Seed Dormancy and Germination by Temperature, Light and Nitrate. Bot. Rev. 2020, 86, 39–75.
- Footitt, S.; Huang, Z.; Clay, H.A.; Mead, A.; Finch-Savage, W.E. Temperature, Light and Nitrate Sensing Coordinate Arabidopsis Seed Dormancy Cycling, Resulting in Winter and Summer Annual Phenotypes. Plant J. 2013, 74, 1003–1015.
- Finch-Savage, W.E.; Cadman, C.S.C.; Toorop, P.E.; Lynn, J.R.; Hilhorst, H.W.M. Seed Dormancy Release in Arabidopsis Cvi by Dry After-Ripening, Low Temperature, Nitrate and Light Shows Common Quantitative Patterns of Gene Expression Directed by Environmentally Specific Sensing. Plant J. Cell Mol. Biol. 2007, 51, 60–78.
- Footitt, S.; Douterelo-Soler, I.; Clay, H.; Finch-Savage, W.E. Dormancy Cycling in Arabidopsis Seeds Is Controlled by Seasonally Distinct Hormone-Signaling Pathways. Proc. Natl. Acad. Sci. USA 2011, 108, 20236–20241.
- Yan, D.; Easwaran, V.; Chau, V.; Okamoto, M.; Ierullo, M.; Kimura, M.; Endo, A.; Yano, R.; Pasha, A.; Gong, Y.; et al. NIN-like Protein 8 Is a Master Regulator of Nitrate-Promoted Seed Germination in Arabidopsis. Nat. Commun. 2016, 7, 13179.
- Leprince, O.; Pellizzaro, A.; Berriri, S.; Buitink, J. Late Seed Maturation: Drying without Dying. J. Exp. Bot. 2017, 68, 827–841.
- Delgado-Sánchez, P.; Ortega-Amaro, M.A.; Jiménez-Bremont, J.F.; Flores, J. Are Fungi Important for Breaking Seed Dormancy in Desert Species? Experimental Evidence in Opuntia streptacantha (Cactaceae): Fungi Break Seed Dormancy in Opuntia. Plant Biol. 2011, 13, 154–159.
- Roberts, E.H. Seed Ageing. By D. A. Priestley. Ithaca and London: Cornell University Press (Comstock Publishing Associates) (1986), pp. 304, $41.25. Exp. Agric. 1987, 23, 227.
- Zinsmeister, J.; Leprince, O.; Buitink, J. Molecular and Environmental Factors Regulating Seed Longevity. Biochem. J. 2020, 477, 305–323.
- Hallam, N.D.; Roberts, B.E.; Osborne, D.J. Embryogenesis and Germination in Rye (Secale Cereale L.): III. Fine Structure and Biochemistry of the Non-Viable Embryo. Planta 1973, 110, 279–290.
- Roberts, B.E.; Payne, P.I.; Osborne, D.J. Protein Synthesis and the Viability of Rye Grains. Loss of Activity of Protein-Synthesizing Systems in Vitro Associated with a Loss of Viability. Biochem. J. 1973, 131, 275–286.
- Benamar, A.; Tallon, C.; Macherel, D. Membrane Integrity and Oxidative Properties of Mitochondria Isolated from Imbibing Pea Seeds after Priming or Accelerated Ageing. Seed Sci. Res. 2003, 13, 35–45.
- Bailly, C.; El-Maarouf-Bouteau, H.; Corbineau, F. From Intracellular Signaling Networks to Cell Death: The Dual Role of Reactive Oxygen Species in Seed Physiology. Comptes Rendus Biol. 2008, 331, 806–814.
- Kranner, I.; Birtić, S.; Anderson, K.M.; Pritchard, H.W. Glutathione Half-Cell Reduction Potential: A Universal Stress Marker and Modulator of Programmed Cell Death? Free Radic. Biol. Med. 2006, 40, 2155–2165.
- Clerkx, E.J.M.; El-Lithy, M.E.; Vierling, E.; Ruys, G.J.; Blankestijn-De Vries, H.; Groot, S.P.C.; Vreugdenhil, D.; Koornneef, M. Analysis of Natural Allelic Variation of Arabidopsis Seed Germination and Seed Longevity Traits between the Accessions Landsberg Erecta and Shakdara, Using a New Recombinant Inbred Line Population. Plant Physiol. 2004, 135, 432–443.
- Long, R.L.; Kranner, I.; Panetta, F.D.; Birtic, S.; Adkins, S.W.; Steadman, K.J. Wet-Dry Cycling Extends Seed Persistence by Re-Instating Antioxidant Capacity. Plant Soil 2011, 338, 511–519.
- Kibinza, S.; Vinel, D.; Côme, D.; Bailly, C.; Corbineau, F. Sunflower Seed Deterioration as Related to Moisture Content during Ageing, Energy Metabolism and Active Oxygen Species Scavenging. Physiol. Plant. 2006, 128, 496–506.
- Waterworth, W.M.; Bray, C.M.; West, C.E. The Importance of Safeguarding Genome Integrity in Germination and Seed Longevity. J. Exp. Bot. 2015, 66, 3549–3558.
- Châtelain, E.; Satour, P.; Laugier, E.; Ly Vu, B.; Payet, N.; Rey, P.; Montrichard, F. Evidence for Participation of the Methionine Sulfoxide Reductase Repair System in Plant Seed Longevity. Proc. Natl. Acad. Sci. USA 2013, 110, 3633–3638.
- Ogé, L.; Bourdais, G.; Bove, J.; Collet, B.; Godin, B.; Granier, F.; Boutin, J.-P.; Job, D.; Jullien, M.; Grappin, P. Protein Repair L -Isoaspartyl Methyltransferase1 Is Involved in Both Seed Longevity and Germination Vigor in Arabidopsis. Plant Cell 2008, 20, 3022–3037.
- Paparella, S.; Araújo, S.S.; Rossi, G.; Wijayasinghe, M.; Carbonera, D.; Balestrazzi, A. Seed Priming: State of the Art and New Perspectives. Plant Cell Rep. 2015, 34, 1281–1293.
- Lutts, S.; Benincasa, P.; Wojtyla, L.; Kubala, S.; Pace, R.; Lechowska, K.; Quinet, M.; Garnczarska, M. Seed Priming: New Comprehensive Approaches for an Old Empirical Technique. In New Challenges in Seed Biology—Basic and Translational Research Driving Seed Technology; Araujo, S., Balestrazzi, A., Eds.; InTech Open Book Series; InTech: Rijeka, Croatia, 2016; pp. 1–46. ISBN 978-953-51-2658-4.
