Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Arturo Evangelista and Version 2 by Camila Xu.
Multimodality imaging is the basis of the diagnosis, follow-up, and surgical management of bicuspid aortic valve (BAV) patients. The aim of this review is to analyze the recent contribution of multimodality imaging in the knowledge and management of bicuspid aortic valve.
- bicuspid aortic valve
- echocardiography
- computed tomography
1. Introduction
Bicuspid aortic valve (BAV) is the most common congenital heart disease, with a reported prevalence of 0.8–1.5% [1][2][1,2] and a male predominance of nearly 3:1. It is considered to be a valvulo-aortopathy characterized by a large individual heterogeneity, both at the valvular and aorta level, but also in the possible associated disorders, complications, and prognoseis [3][4][3,4]. The most frequent complication of the BAV condition in adults is aortic valvular dysfunction, more frequently in the form of severe aortic stenosis (AS), needing surgical aortic repair or AV replacement (AVR) (population-based 25-year risk of AVR is up to 50%) [1]. The second most frequent complication, especially in those above 60 years-old, is ascending aorta dilation. However, the important heterogeneity in terms of the pattern of aortic dilation described in the complete BAV cohort is possible heterogeneity in the molecular, rheologic, and clinical features [5][6][7][8][9][5,6,7,8,9].
2. Diagnosis and Bicuspid Aortic Valve (BAV) V Phenotype
Transthoracic echocardiography (TTE) is the first imaging tool to diagnose the presence of BAV, given its accuracy in 80–90% of cases [1]. Nevertheless, there are some limitations we should take into account, like the extensively-calcified valve, poor visualization of the valve, or the distal ascending aorta. Other imaging techniques such as computed tomography (CT) or cardiac magnetic resonance (CMR) may solve these drawbacks. Transesophageal echocardiography (TEE) is also useful for detecting BAV; however, we must remember it is semi-invasive and it also has some limitations in order to assess the upper part of the ascending aorta and the proximal arch.
The BAV phenotypic expression represents an anatomic continuum of increasing non-fused cusp commissural angles and increasing similarity of cusp size and shape. This spectrum goes from the partial-fusion BAV (very near to a tricuspid aortic valve) to other phenotypes such us those asymmetric fused, symmetric fused phenotypes with and without a raphe, and finally to the two-sinus BAV, which is considered the most severe defect and is anatomically close to perfect “bicuspidity” [10][19]. The best view of the phenotype BAV is the TTE parasternal short-axis or its equivalent in TEE, CT, or CMR (Figure 1).
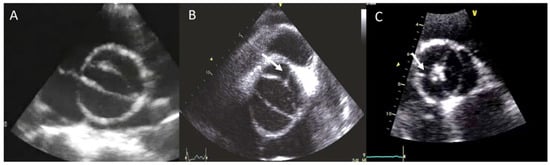

Figure 1. Most frequent types of BAV by TTE. (A) BAV without raphe type antero−posterior; (B) RC−LC fusion with raphe (arrow); (C) RC−NC fusion with raphe (arrow).
The most common type of BAV is the fused one (90–95% of the cases) [10][19]. This is described as two of the three cusps appearing fused within three aortic sinuses, resulting in two functional cusps commonly different in shape and size. A congenital fibrous ridge is often described between the fused cusps, and it is what we know as “raphe”. The most frequent BAV phenotype is the one with right−left (R−L) fusion, considered to be present in 75% of the cohort. This is followed by the right−non-coronary (R−N) fusion (20–25%), and finally the left−non (L−N) fusion (<3%). The two-sinus BAV type is uncommon and accounts for approximately 5–7% of cases. In contrast to the fused type, the two-sinus BAV appearance suggests that two roughly equal size-shape cusps, each cusp occupying 180 degrees of the annular circumference, are “formed” within only two aortic sinuses, resulting in a two-sinus/two-cusp valve [11][20] with a latero−lateral (side-to-side) or antero−posterior (front-and-back) position. The presence of an incomplete fusion of two leaflets (mini-raphe) cannot be easily visualized by TTE [12][21] (Figure 2). In these cases, we should use other techniques such as TEE and CT. This mini-raphe has been considered to be involved in aorta dilation [13][22].
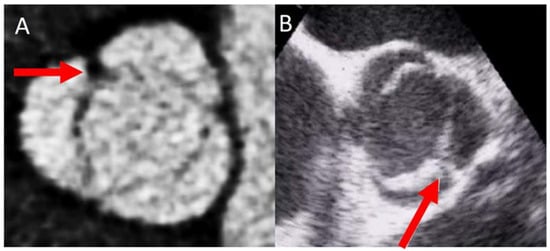

Figure 2.
BAV with partial fusion (forma frustre). Arrows show the mini raphe by CT (
A
) and by TEE (
B
).
In order to solve the limitations of the TTE, CMR and CT are very useful in the morphologic valve evaluation when it is heavily calcified, and can readily assess aorta diameters [14][23]. CT can quantify the valve calcification, which can help clinicians to evaluate valve stenosis severity. The multiplanar reconstructions permit CMR and CT to precisely assess the aortic diameters [15][16][24,25]. Although CT offers a higher spatial resolution, CMR provides information of the valvular dysfunction and left ventricular function. Cine images (SSFP sequences) can be used for measuring the luminogram of the aorta, particularly in the aortic root, owing the valvular plane movement. Aorta diameters should be measured in double oblique technique) [17][26] (Figure 3). Screening for coarctation should be performed in BAV patients, however CMR on the first echo is the preferred technique in younger patients in order to avoid radiation.
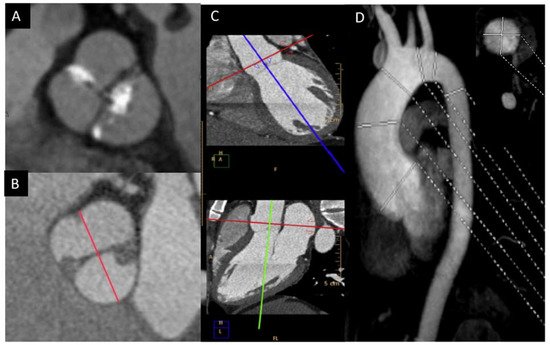

Figure 3. BAV by CT showing left−right fusion with raphe calcification and mild non-coronary sigmoid edge calcification (A); CMR showing two-sinus anteroposterior BAV (B); double obliquity image for measuring the maximum diameter of the aortic root by CT (C); thoracic aorta diameters by angio-CMR sagittal projection; the right upper part shows the aortic root section obtained with double obliquity image (D).
3. Familial Screening
BAV is diagnosed in 4.6–11% of first-degree relatives (FDR) [18][19][20][21][27,28,29,30]. Moreover, the frequency of aorta dilation in FDR is nearly 10% in those with tricuspid aortic valves. In a recent publication by our group, we reported the presence of a mini-raphe by CT in 41% of cases with aorta dilation in FDR and an apparent absence of BAV by TTE [22][31], and further studies demonstrate that, compared with BAV and tricuspid valve patients, the presence of a mini-raphe is associated with ascending aorta flow pattern alterations with increased flow eccentricity and increased vorticity [13][22]. These data are in favor of the screening in the FDR of BAV patients.
4. Valvular Dysfunction
In adult patients with BAV, the most frequent complication is valvular dysfunction, commonly known as aortic stenosis (AS), which necessitates surgical aortic valve replacement (AVR) or repair [3][23][24][3,32,33]. TTE is the technique of choice in the diagnosis and quantification of aortic valve dysfunction. The subtype with a lower prevalence of valvular dysfunction is pure BAV without raphe. This subgroup showed both a lower prevalence of AS, as well as of aortic regurgitation (AR), in a large international registry that included 2118 patients [25][34]. If we differentiate in terms of age, AR has been described to be more frequent in young individuals, and AS more frequent in the older ones [26][35].
4.1. Valve Calcification
BAV calcification has been associated with inflammatory processes mediated by hemodynamic, molecular, and genetic factors [27][36]. Calcification is usually localized in the raphe, and it is considered to be a risk factor for developing valve degeneration and aortic stenosis (AS). Stenotic calcification of a BAV could appear more than ten years earlier than in those with the tricuspid aortic valve. It is related to conventional cardiovascular risk factors. An echocardiographic semiquantitative score for aortic valve calcification has been validated [28][37]. CT calcium scoring is useful for valve stenosis quantification in doubtful cases. In a large crossectional study of 852 BAV [26][35] patients, we found age, arterial hypertension, dyslipidemia, smoking, and the BAV-RN morphotype to be associated with aortic valve calcification. However, CT score was not associated with valve phenotype [29][38].
4.2. Aortic Stenosis
Adults diagnosed with BAV have a clear increased risk of developing AS, usually secondary to leaflet calcification. Previous studies have showed that over 15 years of follow up, 12.3% of the cohort required aortic valve replacement for severe AS [23][32]. In our daily practice, TTE is the most used technique for evaluating the presence of this complication, and for guiding appropriate management. AS quantification is based on the same parameters of tricuspid valves (Figure 4). We must remember that the aortic valve area may be significantly underestimated by TTE owing to underestimation of aortic annulus measurement. However, the use of CTA may correct these underestimations [1].
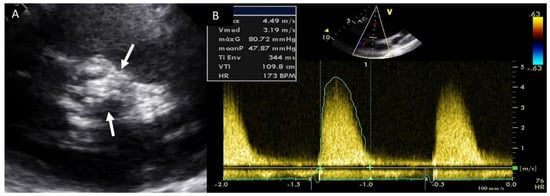

Figure 4. Severe aortic stenosis in BAV with severe calcification (arrows) (A); the mean gradient by continuous-wave Doppler is 48 mmHg (B).
Although in some studies it has been suggested that AS progresses faster in BAV than in the tricuspid aortic valve [30][39], a recent large study by Michelena et al. [31][40] showed that BAV and tricuspid aortic valve stenosis have similar progression rates, with evidence of accelerated progression (non-linearity) in those attaining severe AS. Determinants of rapid progression in BAV-AS, again, are modifiable cardiovascular risk factors, particularly for patients with BAV who are <60 years of age.
4.3. Aortic Regurgitation
Valvular dysfunction as AR is more frequent in younger patients, but also in males [32][41]. Although its prevalence ranges between 47% and 64%, moderate−severe grade appears in less than 30% of individuals [9][23][24][25][26][33][9,32,33,34,35,42]. Annular dilation and cusp prolapse or retraction is the most common underlying cause of chronic regurgitation, acting either alone or in combination [34][43]. The evaluation of AR severity in BAV by TTE is challenging, given that eccentric jets are common. In some cases, CMR may be superior to TTE for quantifying AR (Figure 5) [35][36][44,45]. The CMR phase contrast is accurate and reproducible in AR assessment with the estimation of the regurgitant volume and regurgitant fraction. We labelled a regurgitant fraction of more than 30% as severe [37][46].
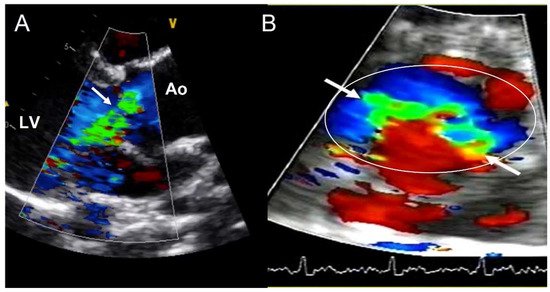

Figure 5. Severe aortic regurgitation in a BAV. (A) Eccentric jet in parasternal long axis-view (arrow). (B) The short-axis view shows the elliptic shape of the regurgitant orifice (arrows) in the aortic annulus (circle). Ao—aorta; LV—left ventricle.
