Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Jessie Wu and Version 1 by Hebah AL Ubeed.
Cannabis is a rich source of phytochemicals with over 125 types of cannabinoids and 400 non-cannabinoids like flavonoids, alkaloids, phenols, and terpenes. These phytochemicals have been linked to various health benefits. Cannabis is well-known for its numerous therapeutic activities, as demonstrated in pre-clinical and clinical studies primarily due to its bioactive compounds. The Cannabis industry is rapidly growing; therefore, product development and extraction methods have become crucial aspects of Cannabis research.
- Cannabis
- medicinal cannabis
- extraction
- cannabinoids
1. Introduction
There has been an increased interest in medical applications of cannabis over the last decades. Cannabis can be classified based on genetics, phenotypic properties, and chemical composition. All these types are rich in bioactive phytochemicals. However, the phytochemical composition varies in different types. For instance, Cannabis sativa or industrial hemp has a higher cannabidiol (CBD) level than Cannabis indica and Cannabis ruderalis, whereas Cannabis indica has a higher level of the psychoactive cannabinoid delta-9-tetrahydrocannabinol (Δ9-THC), and Cannabis ruderalis has a lower level of Δ9-THC as compared to Cannabis sativa [1]. Most of the cannabinoids in Cannabis are derived from cannabigerolic acid (CBGA) via olivetolate geranyl transferase [2]. CBGA is then converted to major cannabinoids, such as Δ9-tetrahydrocannabinolic acid (THCA), cannabidiolic acid (CBDA), and cannabichromenic acid (CBCA) by oxide cyclase enzymes, including THCA synthase, CBDA synthase, and CBCA synthase in trichomes [3]. A nonenzymatic reaction caused by drying, heating, or combustion can further produce other active compounds (Figure 1). For instance, tetrahydrocannabinol (THC) can be converted to Δ8-THC, cannabinol (CBN), and cannabinolic acid (CBNA). CBD and cannabichromene (CBC) can be converted to cannabicyclolic (CBL), cannabicyclolic acid (CBLA), and cannabigerol (CBG) [4].
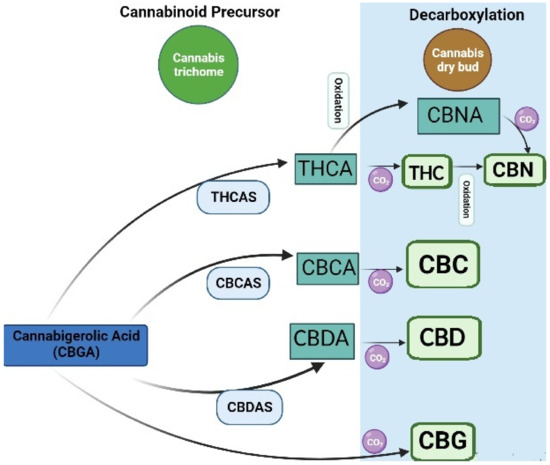
Figure 1. Biosynthesis of cannabinoids: cannabigerolic acid; CBGA, tetrahydrocannabinolic acid synthase; THCAS, cannabidiolic acid synthase; CBDAS, cannabichromene acid synthase; CBCAS, tetrahydrocannabinolic acid, THCA; cannabidiolic acid; CBDA, cannabichromenic acid; CBCA, cannabinolic acid; CBNA, cannabichromene; CBC, cannabidiol; CBD, cannabigerol; CBG, cannabinol; CBN, tetrahydrocannabinol; THC.
Table 1. Extraction of phytochemicals from Cannabis.
| Extraction Technique | Extraction Conditions/Procedures | Advantages and Limitations | References | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Solvent extraction | The plant materials (0.9–1.1 g) were crushed and extracted in 45-mL ethanol for 15 min with the agitation of 400 rpm. Extracts were centrifuged briefly for 30 s at 2000 rpm. The supernatant was collected and filtered. | A simple technique, but not very efficient | [66] | [5] | ||||||||||
| Solvent extraction | Samples were extracted in hexane and ethanol mixture at 7:3 ( | v | / | v | ) and shaken for 45 min at 225 rpm in a TU-400 orbital shaker incubator at room temperature to obtain the extract. | A simple technique, but not very effective | [67] | [6] | ||||||
| Solvent extraction | Samples were extracted in ethanol at room temperature for 45 min to obtain the extract. | A simple technique, but not very effective | [68] | [7] | ||||||||||
| Solvent extraction | The plant material (100 g) was Pulverised and extracted with 500-mL petrol ether acidified with acetic acid (0.5-mL CH | 3 | COOH in 500-mL PE). The filtrated extract was re-extracted 3 times with 400 mL of NaOH and Na | 2 | SO | 3 | (2% each). These combined extracts were acidified with 500 mL of 5% sulfuric acid until pH reached 3 and immediately extracted 3 times with 400-mL TBME. These combined organic extracts were dried with Na | 2 | SO | 4 | , filtrated, and concentrated in a rotary evaporator at 25–30 °C with cryostatic cooling of the vapours. The concentrate was dried overnight at vacuum conditions, yielding 1.71-g brown amorphous material. | A simple technique, but not effective and difficult for commercial production | [69] | [8] |
| Soxhlet extraction | Ground dried samples (2 g) were extracted using Soxhlet extractor for 1, 2, or 3 h with 75 mL of n-hexane or methanol then cooled to room temperature to obtain the extract. | A simple technique, but not effective | [70] | [9] | ||||||||||
| Sonication | The dried and pulverised plant material (50 g) was extracted by sonication and periodic shaking (30 min) with 250-mL petroleum ether, which was acidified with 0.5-mL concentrated acetic acid. The extract was further extracted 3 times with 200 mL of an aqueous solution (2% | w/v | each) of sodium hydroxide and sodium sulphite. The combined and cooled water phases were acidified with about 250-mL cooled sulphuric acid to pH 3 and immediately extracted 5 times with 200-mL diethyl ether. The combined organic phases were dried with sodium sulphate and evaporated to dryness. | Quite effective advanced technique, but it is challenging to apply on a commercial scale | [71] | [10] | ||||||||
| Sonication | Samples (1 g) were extracted with 10 mL of the extraction solution (100 μg/mL of n-Tridecane in ethyl acetate) by sonication for 15 min to obtain the extract. | An advanced technique, but not under optimal conditions | [72] | [11] | ||||||||||
| Ultrasound-assisted extraction (UAE) | A small amount (0.25 g) of the sample was mixed with 10 mL of ethanol and was then extracted 3 times using UAE at 40 °C for 15 min. The solution was then filtered through a paper filter to obtain the final extract. | An advanced technique, but not under optimal conditions | [73] | [12] | ||||||||||
| Pressurized liquid extraction (PLE) | Samples of | Cannabis | (0.3 g) were mixed with sand and then placed into a 22-mL stainless-steel extraction cell with a cellulose filter. The sample cells were then closed and placed into the carousel of the ASE 200 system. Methanol or n-hexane was used as extraction solvents. Extractions were carried out at 25, 50, 75, 100, 125, and 150 °C at a pressure of 40 bar. Extractions were performed for 5, 10, 15, or 20 min. After the extraction process, the extraction cell content was flushed with the same solvent in the amount equal to 60% of the extraction cell volume and purged for 60 s by applying pressurized nitrogen (at 150 psi) to obtain the final extracts. | An advanced technique, but it has not been operated under optimal conditions. | [70] | [9] | ||||||||
| Ultrasound-assisted extraction (UAE) | A small amount (0.25 g) of sample was mixed with 10 mL of ethanol and was then extracted 3 times using UAE at 40 °C for 15 min. The solution was then filtered through a paper filter to obtain the final extract. | An advanced technique, but not under optimal conditions | [73] | [12] | ||||||||||
| Pulse electric field extraction (PEF) | The seeds were treated by the PEF process (0, 3, and 6 kV/cm). The PEF process was conducted with a capacity of a process chamber of 4 L. Maximum voltage of the instrument was 7 Kv, and its capacitance was 8 μF. | An advanced technique, but not under optimal conditions | [ | 74 |
2. Current Techniques for Extraction of Phytochemicals from Cannabis
2.1. Conventional Extraction of Phytochemicals from Cannabis
Cannabis is chemically diverse, with cannabinoids, phenolic compounds, and terpenes being the most important phytochemicals[75][13]. Several conventional extraction techniques have been applied to extract phytochemicals from Cannabis using various solvents [75-83][14]. Different conventional extraction methods, including Soxhlet extraction, maceration, and dynamic maceration, have been employed to extract phytochemicals from Cannabis [68,75,76,80,82][7][13][15][16][17]. However, studies have also revealed the caveats of using conventional extraction methods, such as the extraction of unwanted substances and degradation of heat-sensitive compounds due to extraction under high temperature [84][18]. As cannabinoids, phenolic compounds, and terpenes are the three main classes of secondary metabolites in Cannabis, the conventional extraction of these main classes is discussed further in the following subsections. The extraction of phytochemicals from Cannabis are summarised in Table 1.
2.1.1. Conventional Extraction of Cannabinoids
Cannabis contains 125 individual cannabinoids, but Δ9-THCA and CBDA are the most predominant [81][19]. These two compounds undergo decarboxylation to produce Δ9-THC and CBD [81,85][19][20] (Figure 1). Ethanol has been found as an effective solvent in extracting cannabinoids using hot maceration [68,75][7][13] as well as Soxhlet extraction [86][21]. However, the extraction efficiency of these two conventional techniques was significantly lower than that of the advanced microwave-assisted extraction [86][21]. Although conventional extraction techniques have advantages such as simple procedures, easy operations, and affordability, these conventional techniques have several drawbacks, including longer extraction times and the demand for larger solvent volumes, leading to an inclined overall operation cost and harmful environmental impact compared to modern techniques [80][16]. Furthermore, a stable high temperature of Soxhlet extraction has also been reported to accelerate the degradation of THCA to THC and, finally, to CBN, leading to high levels of THC and CBN in the extract [70,80][9][16]. A recent study extracted CBD from inflorescences of C. sativa using the methanol solvent maceration technique and supercritical fluid extraction technique and found that conventional extraction obtained a higher oil yield but lower CBD in comparison with supercritical fluid extraction [81][19].
Another conventional extraction technique called dynamic maceration has also been applied to extract cannabinoids, mostly from industrial hemp [79,80][22][16]. Dynamic maceration is a solid-lipid extraction procedure where a sample is soaked in organic solvents that were selected based on the polarity of the target compounds at a specific temperature for a specific time and then agitated [79,80][22][16]. Ethanol, acetonitrile, and hexane are the common solvents used to extract cannabinoids [87-89][23]. Ethanol was reported to be more efficient in extracting acidic cannabinoids using dynamic maceration than other organic solvents (hexane, acetone, and methanol); however, for neutral cannabinoids, ethanol is on par with other organic solvents [79][22]. Ethanol was also found to be more effective than other organic solvents in different studies [73,90][12][24]. Furthermore, organic solvent mixtures such as methanol and chloroform at 9:1 (v/v) were effective to recover cannabinoids from C. sativa under constant agitation [91][25]. Interestingly, Romano and Hazekamp [92][26] discovered that olive oil is more effective in extracting Cannabis oils containing cannabinoids and terpenes than ethanol, which also extracts chlorophylls, imparting a distinct green colour and unpleasant taste in the final product. The cannabinoid degradation rate was found to be much slower in olive oil extracts as compared to ethanolic extracts [80,93][16][27].
2.1.2. Conventional Extraction of Phenolic Compounds
Three major classes of phenolic compounds, flavonoids, stilbenoids, and lignans, have been identified in Cannabis [52,75,94][28][13][29]. Acetone, methanol, ethanol, and their aqueous mixtures are the common organic solvents to extract phenolic compounds from industrial hemp. Most of these studies quantified the phenolic compounds of the hemp extracts by the overall determination of the total phenolic content and total flavonoid content. In addition, several studies have quantified individual phenolic compounds, such as caffeic acid, gallic acid, rosmarinic acid, p-OH-benzoic acid, ferulic acid, 3,4-dihydroxybenzoic acid, p-coumaric acid, syringic acid, quercetin, luteolin, canniprene, cannflavin A, cannflavin B, catechin, naringenin, isorhamnetin, resveratrol, rutin trihydrate, apigenin, and apigenin 7-glucoside in the extracts [95-98][30]. Of note, most conventional extraction was conducted at room temperature and not under optimal conditions [99-103][31]. Furthermore, an aqueous solution of 2-hydroxypropyl-β-cyclodextrin (a green solvent) has also been applied to recover phenolic compounds from the by-products of industrial hemp oil processing [103][31].
2.1.3. Conventional Extraction of Terpenes
Terpenes such as α-pinene, β-pinene, β-myrcene, limonene, terpinolene, linalool, α-terpineol, β-caryophyllene, α-humulene, and caryophyllene oxide are known as the major constituents of Cannabis essential oils [72,75][11][13]. Similar to cannabinoids and phenolic compounds, most studies reported in the literature have been performed on the industrial hemp variety of C. sativa [104-106][32]. Distillation techniques such as hydro-distillation and steam distillation have been implemented to extract essential oils (terpenes) from Cannabis. However, they were less effective than the advanced supercritical fluid extraction at a lower temperature [105][33]. Interestingly, the GS–MS analyses in the same study revealed that the steam distillation at 130 °C and hydro-distillation at 110 °C showed 37 and 35 terpenes, respectively, in the essential oil of C. Sativa, whereas essential oil extracted with supercritical fluid at 45 °C showed only 30 terpenes [105][33], indicating the generation of terpenoid artefacts.
Solvent-based (both polar and nonpolar) conventional extraction methods have also been implemented to isolate terpenes from Cannabis [66,67,75,107,108][5][6][13][34][35]. For example, Ibrahim et al. [72][11] used an ethyl acetate, ethanol, methanol, and chloroform/methanol (1:9; v/v) mixture and reported that ethyl acetate was the best solvent to recover terpenes from Cannabis [72][11]. However, mixtures of organic solvents are generally more effective for extracting terpenes from Cannabis than individual solvents. For instance, a study on the inflorescences of C. sativa found that the hexane and ethanol mixture at 7:3 (v/v) is more efficient in extracting terpenes compared to hexane or ethanol alone [67][6].
Fischedick et al. [66][5] used ethanol as the extraction solvent to recover both terpenes and cannabinoids, and 36 compounds were identified in 11 varieties of C. sativa [66][5]. Conventional ethanolic extraction was also used by A. Hazekamp and Fischedick [88][36] to isolate monoterpenes (α–pinene, myrcene, and terpinolene); sesquiterpenes ((E)–caryophyllene and α–humulene) [88][36]; and oxygenated terpenes (guaiol, γ_eudesmol, and α_bisabolol) from marijuana and medical Cannabis inflorescences [82][17].
Six different preparation methods were employed in a recent study to obtain Cannabis oils with high cannabinoids and terpene contents [109][37]. In this study, conventional extraction with olive oil or ethanol, along with steam distillation, was included. In conclusion, it was recommended that Cannabis should be macerated at room temperature to obtain the optimal terpene and cannabinoid yields in Cannabis oil [109][37]. Interestingly, a previous study reported that drying methods also influence the terpene composition of Cannabis extract, and gentle drying with a nitrogen stream can retain monoterpenes and sesquiterpenes in the Cannabis extract [82][17]. Thus, the dehydration of samples before extraction should be optimised to prevent the degradation of terpenes.
In summary, although various conventional extraction techniques have been applied to extract terpenes, cannabinoids, and phenolic compounds from Cannabis, most studies have primarily focused on the industrial hemp variety without optimisation. Therefore, further investigations are needed to optimise the conventional extraction parameters, such as type of solvent, sample-to-solvent ratio, temperature, and time, which could benefit the recovery of these compounds from both industrial hemp and medicinal marijuana. The implementation of mathematical prediction models is also recommended to facilitate the optimisation process to obtain greater yields of bioactive compounds in Cannabis extracts. Impacts of the variations related to season; geographical location; and Cannabis type (strain, chemotypes, and chemovars) on the recovery of these compounds in Cannabis extracts or oils should also be considered while designing future studies.
2.2. Advanced Extraction Techniques
Open-loop and closed-loop systems with butane hash oil (BHO) have been applied to extract terpenes with more flavours and aroma from Cannabis. However, the closed-loop system is often used, as it is much safer and with more advantages than the open-loop setting [110][38]. BHO is one of the cheapest and efficient solvents that offer the most desired final product. However, its most common disadvantage is being hard to handle in bigger batches, as it is highly flammable, colourless, and odourless [111][39]. Therefore, the legal use of BHO is restricted to licensed producers [112][40]. The closed-loop system consists of a butane reservoir, trim tube, evaporation chamber, vacuum pump, recovery pump, vacuum oven, and gas detectors. This system provides a more stable and environmentally friendly platform for dealing with volatile BHO.
A multi-solvent extraction system, such as PX 40, has been applied to effectively extract phytochemicals from Cannabis. PX 40 typically functions using either butane or propane or a mixture of both. Despite its high cost, it is a productive system [113][41]. In addition, the pressurised liquid extraction (PLE) technique has been applied to extract phytochemicals from Cannabis. Common solvents like water, methanol, acetone, and hexane have been applied to extract the phytochemicals for a short time under high temperature and pressure (temperature range of 75–150 °C and pressure of usually 10.4 MPa). In this technique, when increasing the temperature, the internal pressure in the cell is consequently increased and push the components to the outside of the cell through the cell wall pores [114][42]. Subcritical carbon dioxide (CO2) extraction systems with specialised pressure and controlled temperature have also been applied to extract high-quality cannabinoids, terpenes, and flavonoids. This technique is an effective nonpolar extraction method [115][43]. In addition, carbon (IV) oxide is also an effective method to extract CBD oil, with up to a 90% extraction efficiency [116][44].
Supercritical fluid extraction with CO2 (SFE-CO2) has been applied to extract phytochemicals from Cannabis. This technique is more effective compared to subcritical CO2 extraction systems [115][43]. It should be noted that solvents used and other factors, such as temperature, pressure, and sample types, can affect the extraction yield of this technique. The best conditions for extracting phytochemicals were found with a back pressure of 12 MPa, a flow rate of 10 mL/min CO2, and a pump rate of 1 mL/min, with a temperature of 25 °C. The solvent gradient conditions were 100−50% solvent A and 0−50% solvent B, and the time was 0–25 min, and all the extractions were run in two cycles [117-119][45]. When scaling up in a pilot-scale using SFE-CO2 (SCF100 model 3 PLC-GR-DLMP, Separeco S.r.l, Pinerolo, Italy), 10 MPa pressure and a temperature of 40 °C were the best conditions [120][46].
Other advanced extraction techniques, such as dynamic maceration (DM), ultrasound-assisted extraction (UAE), and microwave-assisted extraction (MAE), have been applied to extract phytochemicals from Cannabis [73][12]. The DM technique focuses on maceration of the Cannabis in organic solvent, then concentrating the extracted solution by removing the solvent under reduced pressure, high temperature, and acid. However, the chemical structures of the final target compounds can change during the extraction process [68][7].
MAE and UAE have been found to effectively extract phytochemicals from Cannabis [73,121][12][47]. Different factors can influence the extraction efficiency of phytochemicals from Cannabis using UAE and MAE, such as the type of solvent, sample size, sample-to-solvent ratio, time, and power. However, none of the previous studies comprehensively compared and established the optimal conditions for these two techniques when extracting cannabinoids, phenolics, or terpenes from Cannabis. The extraction of phytochemicals from Cannabis are summarised in Table 1.
References
- Gloss, D. An overview of products and bias in research. Neurotherapeutics 2015, 12, 731–734.
- Fellermeier, M.; Eisenreich, W.; Bacher, A.; Zenk, M.H. Biosynthesis of cannabinoids. Incorporation experiments with (13)c-labeled glucoses. Eur. J. Biochem. 2001, 268, 1596–1604.
- Taura, F.; Sirikantaramas, S.; Shoyama, Y.; Yoshikai, K.; Shoyama, Y.; Morimoto, S. Cannabidiolic-acid synthase, the chemotype-determining enzyme in the fiber-type Cannabis sativa. FEBS Lett. 2007, 581, 2929–2934.
- Thomas, B.F.; Elsohly, M. The Analytical Chemistry of Cannabis: Quality Assessment, Assurance, and Regulation of Medicinal Marijuana and Cannabinoid Preparations; Elsevier: Amsterdam, The Netherlands, 2015.
- Fischedick, J.T.; Hazekamp, A.; Erkelens, T.; Choi, Y.H.; Verpoorte, R. Metabolic fingerprinting of Cannabis sativa L., cannabinoids and terpenoids for chemotaxonomic and drug standardization purposes. Phytochemistry 2010, 71, 2058–2073.
- Namdar, D.; Mazuz, M.; Ion, A.; Koltai, H. Variation in the compositions of cannabinoid and terpenoids in Cannabis sativa derived from inflorescence position along the stem and extraction methods. Ind. Crops Prod. 2018, 113, 376–382.
- Ramirez, C.L.; Fanovich, M.A.; Churio, M.S. Chapter 4—cannabinoids: Extraction methods, analysis, and physicochemical characterization. In Studies in Natural Products Chemistry; Attaur, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 61, pp. 143–173.
- Dussy, F.E.; Hamberg, C.; Luginbühl, M.; Schwerzmann, T.; Briellmann, T.A. Isolation of δ9-thca-a from hemp and analytical aspects concerning the determination of δ9-thc in cannabis products. Forensic Sci. Int. 2005, 149, 3–10.
- Wianowska, D.; Dawidowicz, A.L.; Kowalczyk, M. Transformations of tetrahydrocannabinol, tetrahydrocannabinolic acid and cannabinol during their extraction from Cannabis sativa L. J. Anal. Chem. 2015, 70, 920–925.
- Lehmann, T.; Brenneisen, R. A new chromatographic method for the isolation of (−)-δ9-(trans)-tetrahydrocannabinolic acid a. Phytochem. Anal. 1992, 3, 88–90.
- Ibrahim, E.A.; Wang, M.; Radwan, M.M.; Wanas, A.S.; Majumdar, C.G.; Avula, B.; Wang, Y.-H.; Khan, I.A.; Chandra, S.; Lata, H. Analysis of terpenes in Cannabis sativa L. Using gc/ms: Method development, validation, and application. Planta Med. 2019, 85, 431–438.
- Brighenti, V.; Pellati, F.; Steinbach, M.; Maran, D.; Benvenuti, S. Development of a new extraction technique and hplc method for the analysis of non-psychoactive cannabinoids in fibre-type Cannabis sativa L. (hemp). J. Pharm. Biomed. Anal. 2017, 143, 228–236.
- Haji-Moradkhani, A.; Rezaei, R.; Moghimi, M. Optimization of pulsed electric field-assisted oil extraction from cannabis seeds. J. Food Process. Eng. 2019, 42, e13028.
- Isidore, E.; Karim, H.; Ioannou, I. Extraction of phenolic compounds and terpenes from Cannabis sativa L. By-products: From conventional to intensified processes. Antioxidants 2021, 10, 942.
- Nuapia, Y.; Tutu, H.; Chimuka, L.; Cukrowska, E. Selective extraction of cannabinoid compounds from cannabis seed using pressurized hot water extraction. Molecules 2020, 25, 1335.
- Addo, P.W.; Brousseau, V.D.; Morello, V.; MacPherson, S.; Paris, M.; Lefsrud, M. Cannabis chemistry, post-harvest processing methods and secondary metabolite profiling: A review. Ind. Crops Prod. 2021, 170, 113743.
- Lazarjani, M.P.; Young, O.; Kebede, L.; Seyfoddin, A. Processing and extraction methods of medicinal cannabis: A narrative review. J. Cannabis Res. 2021, 3, 32.
- Micalizzi, G.; Vento, F.; Alibrando, F.; Donnarumma, D.; Dugo, P.; Mondello, L. Cannabis sativa L.: A comprehensive review on the analytical methodologies for cannabinoids and terpenes characterization. J. Chromatogr. 2021, 1637, 461864.
- Radoiu, M.; Kaur, H.; Bakowska-Barczak, A.; Splinter, S. Microwave-assisted industrial scale cannabis extraction. Technologies 2020, 8, 45.
- Marzorati, S.; Friscione, D.; Picchi, E.; Verotta, L. Cannabidiol from inflorescences of Cannabis sativa L.: Green extraction and purification processes. Ind. Crops Prod. 2020, 155, 112816.
- Sirikantaramas, S.; Taura, F. Cannabinoids: Biosynthesis and biotechnological applications. In Cannabis sativa L.—Botany and Biotechnology; Springer: Berlin/Heidelberg, Germany, 2017; pp. 183–206.
- Lewis-Bakker, M.M.; Yang, Y.; Vyawahare, R.; Kotra, L.P. Extractions of medical cannabis cultivars and the role of decarboxylation in optimal receptor responses. Cannabis Cannabinoid Res. 2019, 4, 183–194.
- Fathordoobady, F.; Singh, A.; Kitts, D.D.; Singh, A.P. Hemp (Cannabis sativa L.) extract: Anti-microbial properties, methods of extraction, and potential oral delivery. Food Rev. Int. 2019, 35, 664–684.
- Ciolino, L.A.; Ranieri, T.L.; Taylor, A.M. Commercial cannabis consumer products part 1: Gc–ms qualitative analysis of cannabis cannabinoids. Forensic Sci. Int. 2018, 289, 429–437.
- Stefanidou, M.; Athanaselis, S.; Alevisopoulos, G.; Papoutsis, J.; Koutselinis, A. Δ9-tetrahydrocannabinol content in cannabis plants of greek origin. Chem. Pharm. Bull. 2000, 48, 743–745.
- Namdar, D.; Charuvi, D.; Ajjampura, V.; Mazuz, M.; Ion, A.; Kamara, I.; Koltai, H. Led lighting affects the composition and biological activity of Cannabis sativa secondary metabolites. Ind. Crops Prod. 2019, 132, 177–185.
- Hazekamp, A. Cannabis: Extracting the Medicine; Leiden University: Leiden, The Netherlands, 2007; pp. 1–188.
- Romano, L.L.; Hazekamp, A. Cannabis oil: Chemical evaluation of an upcoming cannabis-based medicine. Cannabinoids 2013, 1, 1–11.
- Citti, C.; Ciccarella, G.; Braghiroli, D.; Parenti, C.; Vandelli, M.A.; Cannazza, G. Medicinal cannabis: Principal cannabinoids concentration and their stability evaluated by a high performance liquid chromatography coupled to diode array and quadrupole time of flight mass spectrometry method. J. Pharm. Biomed. Anal. 2016, 128, 201–209.
- Pollastro, F.; Minassi, A.; Fresu, L.G. Cannabis phenolics and their bioactivities. Curr. Med. Chem. 2018, 25, 1160–1185.
- Izzo, L.; Castaldo, L.; Narváez, A.; Graziani, G.; Gaspari, A.; Rodríguez-Carrasco, Y.; Ritieni, A. Analysis of phenolic compounds in commercial Cannabis sativa L. Inflorescences using uhplc-q-orbitrap hrms. Molecules 2020, 25, 631.
- Allegrone, G.; Pollastro, F.; Magagnini, G.; Taglialatela-Scafati, O.; Seegers, J.; Koeberle, A.; Werz, O.; Appendino, G. The bibenzyl canniprene inhibits the production of pro-inflammatory eicosanoids and selectively accumulates in some Cannabis sativa strains. J. Nat. Prod. 2017, 80, 731–734.
- Teh, S.-S.; Bekhit, A.E.-D.; Birch, J. Antioxidative polyphenols from defatted oilseed cakes: Effect of solvents. Antioxidants 2014, 3, 67–80.
- Ahmed, M.; Ji, M.; Qin, P.; Gu, Z.; Liu, Y.; Sikandar, A.; Iqbal, M.; Javeed, A. Phytochemical screening, total phenolic and flavonoids contents and antioxidant activities of Citrullus colocynthis L. and Cannabis sativa L. Appl. Ecol. Environ. Res. 2019, 17, 6961–6979.
- Mourtzinos, I.; Menexis, N.; Iakovidis, D.; Makris, D.P.; Goula, A. A green extraction process to recover polyphenols from byproducts of hemp oil processing. Recycling 2018, 3, 15.
- Fiorini, D.; Molle, A.; Nabissi, M.; Santini, G.; Benelli, G.; Maggi, F. Valorizing industrial hemp (Cannabis sativa L.) by-products: Cannabidiol enrichment in the inflorescence essential oil optimizing sample pre-treatment prior to distillation. Ind. Crops Prod. 2019, 128, 581–589.
- Zheljazkov, V.D.; Sikora, V.; Semerdjieva, I.B.; Kačániová, M.; Astatkie, T.; Dincheva, I. Grinding and fractionation during distillation alter hemp essential oil profile and its antimicrobial activity. Molecules 2020, 25, 3943.
- Naz, S.; Hanif, M.A.; Bhatti, H.N.; Ansari, T.M. Impact of supercritical fluid extraction and traditional distillation on the isolation of aromatic compounds from cannabis indica and Cannabis sativa. J. Essent. Oil Bear. Plants 2017, 20, 175–184.
- Booth, J.K.; Page, J.E.; Bohlmann, J. Terpene synthases from Cannabis sativa. PLoS ONE 2017, 12, e0173911.
- Casano, S.; Grassi, G.; Martini, V.; Michelozzi, M. Variations in Terpene Profiles of Different Strains of Cannabis sativa L. In Proceedings of the XXVIII International Horticultural Congress on Science and Horticulture for People (IHC2010), A New Look at Medicinal and 925, Lisbon, Portugal, 22–27 August 2010; pp. 115–121.
- Hazekamp, A.; Fischedick, J.T. Cannabis from cultivar to chemovar. Drug Test. Anal. 2012, 4, 660–667.
- Ternelli, M.; Brighenti, V.; Anceschi, L.; Poto, M.; Bertelli, D.; Licata, M.; Pellati, F. Innovative methods for the preparation of medical cannabis oils with a high content of both cannabinoids and terpenes. J. Pharm. Biomed. Anal. 2020, 186, 113296.
- Rosenthal, E.; Bho, G. Butane hash oil-shatter, wax, sauce and beyond, quick american. In Beyond Buds: Next Generation-marijuana Extracts and Cannabis Infusions; Blythe, D.T., Ed.; Quick American: Piedmont, CA, USA, 2018; pp. 87–115.
- Pubchem Compound Summary for Cid 7843, Butane. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Butane (accessed on 1 January 2022).
- Stogner, J.M.; Miller, B.L.J.S. The dabbing dilemma: A call for research on butane hash oil and other alternate forms of cannabis use. Subst. Abus. 2015, 36, 393–395.
- Gegax, K. Gegax Hemp Financial Research; California State University San Marcos: San Marcos, CA, USA, 2020.
- Mustafa, A.; Turner, C. Pressurized liquid extraction as a green approach in food and herbal plants extraction: A review. Anal. Chim. Acta 2011, 703, 8–18.
- Rosenthal, E.; Zeman, G. CO2 extraction. In Beyond Buds: Next Generation-Marijuana Extracts and Cannabis Infusions; Rolph Blythe, D.T., Ed.; Quick American: Piedmont, CA, USA, 2018; pp. 149–167.
- Perrotin-Brunel, H. Sustainable Production of Cannabinoids with Supercritical Carbon Dioxide Technologies; University of Technology of Compiègne Geboren te Rouen: Rouen, France, 2011.
- Hanuš, L.O.; Meyer, S.M.; Muñoz, E.; Taglialatela-Scafati, O.; Appendino, G. Phytocannabinoids: A unified critical inventory. Nat. Prod. Rep. 2016, 33, 1357–1392.
- Lucas, P.G. Regulating compassion: An overview of Canada’s federal medical cannabis policy and practice. Harm Reduct. J. 2008, 5, 1–13.
- Da Porto, C.; Decorti, D.; Natolino, A. Separation of aroma compounds from industrial hemp inflorescences (Cannabis sativa L.) by supercritical CO2 extraction and on-line fractionation. Ind. Crops Prod. 2014, 58, 99–103.
- Cravotto, G.; Boffa, L.; Mantegna, S.; Perego, P.; Avogadro, M.; Cintas, P. Improved extraction of vegetable oils under high-intensity ultrasound and/or microwaves. Ultrason. Sonochem. 2008, 15, 898–902.
More
