Cognitive impairment (CI) is common in heart failure (HF). Patients with HF demonstrate reduced global cognition as well as deficits in multiple cognitive domains compared to controls. Degree of CI may be related to HF severity. HF has also been associated with an increased risk of dementia. Anatomical brain changes have been observed in patients with HF, including grey matter atrophy and increased white matter lesions. Patients with HF and CI have poorer functional independence and self-care, more frequent rehospitalisations as well as increased mortality. Pathophysiological pathways linking HF and CI have been proposed, including cerebral hypoperfusion and impaired cerebrovascular autoregulation, systemic inflammation, proteotoxicity and thromboembolic disease.
- heart failure
- cognitive impairment
- dementia
- cerebral haemodynamics
1. Cognitive Changes in HF
1.1. HF Severity and Degree of CI
1.2. The Impact of Ejection Fraction on CI
1.3. Potential Confounders in the Association between HF and CI
2. Proposed Aetiologies of CI in HF
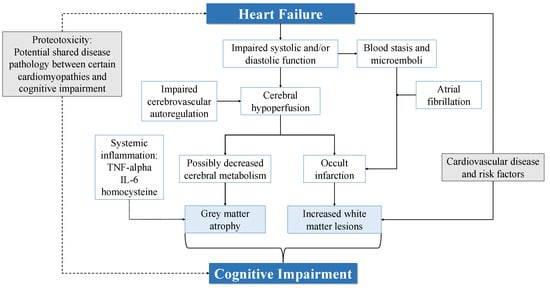
2.1. Cerebral Hypoperfusion and Impaired Autoregulation
2.2. Systemic Inflammation
2.3. Proteotoxicity
2.4. Thromboembolic Disease and Cerebral Infarction
References
- Connors, E.J.; Hauson, A.O.; Barlet, B.D.; Sarkissians, S.; Stelmach, N.P.; Walker, A.D.; Nemanim, N.M.; Greenwood, K.L.; Chesher, N.J.; Wollman, S.C.; et al. Neuropsychological Assessment and Screening in Heart Failure: A Meta-Analysis and Systematic Review. Neuropsychol. Rev. 2021, 31, 312–330.
- Sterling, M.R.; Jannat-Khah, D.; Bryan, J.; Banerjee, S.; McClure, L.A.; Wadley, V.G.; Unverzagt, F.W.; Levitan, E.B.; Goyal, P.; Peterson, J.C.; et al. The Prevalence of Cognitive Impairment Among Adults with Incident Heart Failure: The “Reasons for Geographic and Racial Differences in Stroke” (REGARDS) Study. J. Card. Fail. 2019, 25, 130–136.
- Hammond, C.A.; Blades, N.J.; Chaudhry, S.I.; Dodson, J.A.; Longstreth, W.T.J.; Heckbert, S.R.; Psaty, B.M.; Arnold, A.M.; Dublin, S.; Sitlani, C.M.; et al. Long-Term Cognitive Decline After Newly Diagnosed Heart Failure: Longitudinal Analysis in the CHS (Cardiovascular Health Study). Circ. Heart Fail. 2018, 11, e004476.
- Wolters, F.J.; Segufa, R.A.; Darweesh, S.K.L.; Bos, D.; Ikram, M.A.; Sabayan, B.; Hofman, A.; Sedaghat, S. Coronary heart disease, heart failure, and the risk of dementia: A systematic review and meta-analysis. Alzheimers Dement. 2018, 14, 1493–1504.
- Adelborg, K.; Horváth-Puhó, E.; Ording, A.; Pedersen, L.; Sørensen, H.T.; Henderson, V.W. Heart failure and risk of dementia: A Danish nationwide population-based cohort study. Eur. J. Hear. Fail. 2016, 19, 253–260.
- Qiu, C.; Winblad, B.; Marengoni, A.; Klarin, I.; Fastbom, J.; Fratiglioni, L. Heart failure and risk of dementia and Alzheimer disease: A population-based cohort study. Arch. Intern. Med. 2006, 166, 1003–1008.
- Jefferson, A.L.; Beiser, A.S.; Himali, J.J.; Seshadri, S.; O’Donnell, C.J.; Manning, W.J.; Wolf, P.A.; Au, R.; Benjamin, E. P3-136: Low cardiac index is associated with incident dementia and Alzheimer disease: The Framingham Heart Study. Circulation 2015, 131, 1333–1339.
- Pressler, S.J.; Subramanian, U.; Kareken, D.; Perkins, S.M.; Gradus-Pizlo, I.; Sauvé, M.J.; Ding, Y.; Kim, J.; Sloan, R.; Jaynes, H.; et al. Cognitive Deficits in Chronic Heart Failure. Nurs. Res. 2010, 59, 127–139.
- Harkness, K.; Demers, C.; Heckman, G.A.; McKelvie, R.S. Screening for cognitive deficits using the Montreal cognitive as-sessment tool in outpatients >=65 years of age with heart failure. Am. J. Cardiol. 2011, 107, 1203–1207.
- Hanon, O.; Vidal, J.-S.; de Groote, P.; Galinier, M.; Isnard, R.; Logeart, D.; Komajda, M. Prevalence of Memory Disorders in Ambulatory Patients Aged ≥70 Years with Chronic Heart Failure (from the EFICARE Study). Am. J. Cardiol. 2014, 113, 1205–1210.
- Lee, T.C.; Qian, M.; Liu, Y.; Graham, S.; Mann, D.L.; Nakanishi, K.; Teerlink, J.R.; Lip, G.Y.H.; Freudenberger, R.S.; Sacco, R.L.; et al. Cognitive Decline Over Time in Patients with Systolic Heart Failure: Insights From WARCEF. JACC Heart Fail. 2019, 7, 1042–1053.
- Arslanian-Engoren, C.; Giordani, B.J.; Algase, D.; Schuh, A.; Lee, C.; Moser, D.K. Cognitive dysfunction in older adults hos-pitalized for acute heart failure. J. Card. Fail. 2014, 20, 669–678.
- Kindermann, I.; Fischer, D.; Karbach, J.; Link, A.; Walenta, K.; Barth, C.; Ukena, C.; Mahfoud, F.; Kollner, V.; Kindermann, M.; et al. Cognitive function in patients with decompensated heart failure: The Cognitive Impairment in Heart Failure (CogImpair-HF) study. Eur. J. Heart Fail. 2012, 14, 404–413.
- Huijts, M.; Van Oostenbrugge, R.J.; Duits, A.; Burkard, T.; Muzzarelli, S.; Maeder, M.T.; Schindler, R.; Pfisterer, M.E.; Rocca, H.-P.B.-L. Cognitive impairment in heart failure: Results from the Trial of Intensified versus standard Medical therapy in Elderly patients with Congestive Heart Failure (TIME-CHF) randomized trial. Eur. J. Hear. Fail. 2013, 15, 699–707.
- Emery, A.; Wells, J.; Klaus, S.P.; Mather, M.; Pessoa, A.; Pendlebury, S.T. Underestimation of Cognitive Impairment in Older Inpatients by the Abbreviated Mental Test Score versus the Montreal Cognitive Assessment: Cross-Sectional Observational Study. Dement. Geriatr. Cogn. Disord. Extra 2020, 10, 205–215.
- Hall, C. NT-ProBNP: The mechanism behind the marker. J. Card. Fail. 2005, 11, S81–S83.
- Rørth, R.; Jhund, P.S.; Yilmaz, M.B.; Kristensen, S.L.; Welsh, P.; Desai, A.S.; Køber, L.; Prescott, M.F.; Rouleau, J.L.; Solomon, S.D. Comparison of BNP and NT-proBNP in Patients with Heart Failure and Reduced Ejection Fraction. Circ. Hear. Fail. 2020, 13, e006541.
- Hurk, K.V.D.; Reijmer, Y.D.; Berg, E.V.D.; Alssema, M.; Nijpels, G.; Kostense, P.J.; Stehouwer, C.D.; Paulus, W.J.; Kamp, O.; Dekker, J.M.; et al. Heart failure and cognitive function in the general population: The Hoorn Study. Eur. J. Hear. Fail. 2011, 13, 1362–1369.
- Park, C.M.; Williams, E.D.; Chaturvedi, N.; Tillin, T.; Stewart, R.J.; Richards, M.; Shibata, D.; Mayet, J.; Hughes, A.D. Associ-ations Between Left Ventricular Dysfunction and Brain Structure and Function: Findings from the SABRE (Southall and Brent Revisited) Study. J. Am. Heart Assoc. 2017, 6, e004898.
- Nagata, T.; Ohara, T.; Hata, J.; Sakata, S.; Furuta, Y.; Yoshida, D.; Honda, T.; Hirakawa, Y.; Ide, T.; Kanba, S.; et al. NT-proBNP and Risk of Dementia in a General Japanese Elderly Population: The Hisayama Study. J. Am. Heart Assoc. 2019, 8, e011652.
- Zuccalà, G.; Cattel, C.; Manes-Gravina, E.; Di Niro, M.G.; Cocchi, A.; Bernabei, R. Left ventricular dysfunction: A clue to cog-nitive impairment in older patients with heart failure. J. Neurol. Neurosurg. Psychiatry 1997, 63, 509–512.
- Shin, M.-S.; Lan, S.J.; Kim, S.; Shim, J.L.; Park, J.-K.; Kim, J. Concomitant diastolic dysfunction further interferes with cognitive performance in moderate to severe systolic heart failure. PLoS ONE 2017, 12, e0184981.
- Festa, J.R.; Jia, X.; Cheung, K.; Marchidann, A.; Schmidt, M.; Shapiro, P.A.; Mancini, D.M.; Naka, Y.; Deng, M.; Lantz, E.R.; et al. Association of Low Ejection Fraction with Impaired Verbal Memory in Older Patients With Heart Failure. Arch. Neurol. 2011, 68, 1021–1026.
- Carey, B.J.; Eames, P.J.; Blake, M.J.; Panerai, R.B.; Potter, J.F. Dynamic Cerebral Autoregulation Is Unaffected by Aging. Stroke 2000, 31, 2895–2900.
- Román, G.C. Brain hypoperfusion: A critical factor in vascular dementia. Neurol. Res. 2004, 26, 454–458.
- Athilingam, P.; D’Aoust, R.; Miller, L.; Chen, L. Cognitive Profile in Persons with Systolic and Diastolic Heart Failure. Congest. Hear. Fail. 2012, 19, 44–50.
- Bratzke-Bauer, L.C.; Pozehl, B.J.; Paul, S.M.; Johnson, J.K. Neuropsychological Patterns Differ by Type of Left Ventricle Dys-function in Heart Failure. Arch. Clin. Neuropsychol. 2012, 28, 114–124.
- Vogels, R.L.C.; Oosterman, J.M.; Van Harten, B.; Scheltens, P.; Van Der Flier, W.M.; Schroeder-Tanka, J.M.; Weinstein, H.C. Profile of Cognitive Impairment in Chronic Heart Failure. J. Am. Geriatr. Soc. 2007, 55, 1764–1770.
- Trojano, L.; Incalzi, R.A.; Acanfora, D.; Picone, C.; Mecocci, P.; Rengo, F. Cognitive impairment: A key feature of congestive heart failure in the elderly. J. Neurol. 2003, 250, 1456–1463.
- Almeida, O.P.; Garrido, G.J.; Etherton-Beer, C.; Lautenschlager, N.T.; Arnolda, L.; Flicker, L. Cognitive and brain changes associated with ischaemic heart disease and heart failure. Eur. Hear. J. 2012, 33, 1769–1776.
- Almeida, O.P.; Beer, C.; Lautenschlager, N.T.; Arnolda, L.; Alfonso, H.; Flicker, L. Two-year course of cognitive function and mood in adults with congestive heart failure and coronary artery disease: The Heart-Mind Study. Int. Psychogeriatrics 2011, 24, 38–47.
- Vogels, R.L.; van der Flier, W.; Van Harten, B.; Gouw, A.A.; Scheltens, P.; Schroeder-Tanka, J.M.; Weinstein, H.C. Brain magnetic resonance imaging abnormalities in patients with heart failure. Eur. J. Hear. Fail. 2007, 9, 1003–1009.
- Jefferson, A.L.; Himali, J.J.; Beiser, A.S.; Au, R.; Massaro, J.M.; Seshadri, S.; Gona, P.; Salton, C.J.; DeCarli, C.; O’Donnell, C.J.; et al. Cardiac index is associated with brain aging: The framingham heart study. Circulation 2010, 122, 690–697.
- Alosco, M.L.; Brickman, A.M.; Spitznagel, M.B.; Garcia, S.L.; Narkhede, A.; Griffith, E.Y.; Raz, N.; Cohen, R.; Sweet, L.H.; Colbert, L.H.; et al. Cerebral perfusion is associated with white matter hyperintensities in older adults with heart failure. Congest. Hear. Fail. 2013, 19, E29–E34.
- Choi, B.-R.; Kim, J.S.; Yang, Y.J.; Park, K.-M.; Lee, C.W.; Kim, Y.-H.; Hong, M.-K.; Song, J.-K.; Park, S.-W.; Park, S.-J. Factors Associated with Decreased Cerebral Blood Flow in Congestive Heart Failure Secondary to Idiopathic Dilated Cardiomyopathy. Am. J. Cardiol. 2006, 97, 1365–1369.
- Babayiğit, E.; Murat, S.; Mert, K.U.; Çavuşoğlu, Y. Assesment of Cerebral Blood Flow Velocities with Transcranial Doppler Ultrasonography in Heart Failure Patients with Reduced Ejection Fraction. J. Stroke Cerebrovasc. Dis. 2021, 30, 105706.
- Alosco, M.L.; Spitznagel, M.B.; Cohen, R.; Raz, N.; Sweet, L.H.; Josephson, R.; Hughes, J.; Rosneck, J.; Gunstad, J. Reduced cerebral perfusion predicts greater depressive symptoms and cognitive dysfunction at a 1-year follow-up in patients with heart failure. Int. J. Geriatr. Psychiatry 2013, 29, 428–436.
- Kure, C.E.; Rosenfeldt, F.L.; Scholey, A.; Pipingas, A.; Kaye, D.M.; Bergin, P.J.; Croft, K.; Wesnes, K.; Myers, S.P.; Stough, C. Relationships Among Cognitive Function and Cerebral Blood Flow, Oxidative Stress, and Inflammation in Older Heart Failure Patients. J. Card. Fail. 2016, 22, 548–559.
- Suzuki, H.; Matsumoto, Y.; Ota, H.; Sugimura, K.; Takahashi, J.; Ito, K.; Miyata, S.; Furukawa, K.; Arai, H.; Fukumoto, Y.; et al. Hippocampal Blood Flow Abnormality Associated with Depressive Symptoms and Cognitive Impairment in Patients With Chronic Heart Failure. Circ. J. 2016, 80, 1773–1780.
- van Hout, M.J.P.; Dekkers, I.A.; Westenberg, J.J.M.; Schalij, M.J.; Scholte, A.J.H.A.; Lamb, H.J. Associations between left ventricular function, vascular function and measures of cerebral small vessel disease: A cross-sectional magnetic resonance imaging study of the UK Biobank. Eur. Radiol. 2021, 31, 5068–5076.
- Triantafyllidi, H.; Arvaniti, C.; Lekakis, J.; Ikonomidis, I.; Siafakas, N.; Tzortzis, S.; Trivilou, P.; Zerva, L.; Stamboulis, E.; Kremastinos, D.T. Cognitive Impairment Is Related to Increased Arterial Stiffness and Microvascular Damage in Patients with Never-Treated Essential Hypertension. Am. J. Hypertens. 2009, 22, 525–530.
- van Exel, E.; de Craen, A.J.; Remarque, E.J.; Gussekloo, J.; Houx, P.; der Wiel, A.B.-V.; Frolich, M.; Macfarlane, P.W.; Blauw, G.J.; Westendorp, R.G. Interaction of atherosclerosis and inflammation in elderly subjects with poor cognitive function. Neurology 2003, 61, 1695–1701.
- Sabayan, B.; van Buchem, M.A.; Sigurdsson, S.; Zhang, Q.; Harris, T.B.; Gudnason, V.; Arai, A.E.; Launer, L.J. Cardiac he-modynamics are linked with structural and functional features of brain aging: The age, gene/environment susceptibility (AG-ES)-Reykjavik Study. J. Am. Heart Assoc. 2015, 4, e001294.
- Yun, M.; Nie, B.; Wen, W.; Zhu, Z.; Liu, H.; Nie, S.; Lanzenberger, R.; Wei, Y.; Hacker, M.; Shan, B.; et al. Assessment of cerebral glucose metabolism in patients with heart failure by 18F-FDG PET/CT imaging. J. Nucl. Cardiol. 2020, 1–13.
- Kumar, R.; Yadav, S.K.; Palomares, J.A.; Park, B.; Joshi, S.H.; Ogren, J.A.; Macey, P.M.; Fonarow, G.C.; Harper, R.M.; Woo, M.A. Reduced Regional Brain Cortical Thickness in Patients with Heart Failure. PLoS ONE 2015, 10, e0126595.
- Moody, D.M.; Bell, M.A.; Challa, V.R. Features of the cerebral vascular pattern that predict vulnerability to perfusion or oxy-genation deficiency: An anatomic study. Am. J. Neuroradiol. 1990, 11, 431–439.
- Leeuwis, A.E.; Hooghiemstra, A.M.; Bron, E.E.; Kuipers, S.; Oudeman, E.A.; Kalay, T.; Rocca, H.B.; Kappelle, L.J.; Van Oostenbrugge, R.J.; Greving, J.P.; et al. Cerebral blood flow and cognitive functioning in patients with disorders along the heart–brain axis. Alzheimer’s Dementia: Transl. Res. Clin. Interv. 2020, 6.
- Gruhn, N.; Larsen, F.S.; Boesgaard, S.; Knudsen, G.M.; Mortensen, S.A.; Thomsen, G.; Aldershvile, J. Cerebral Blood Flow in Patients with Chronic Heart Failure Before and After Heart Transplantation. Stroke 2001, 32, 2530–2533.
- Roman, D.D.; Kubo, S.H.; Ormaza, S.; Francis, G.S.; Bank, A.J.; Shumway, S.J. Memory improvement following cardiac transplantation. J. Clin. Exp. Neuropsychol. 1997, 19, 692–697.
- Vorovich, E.; Andrei, A.-C.; Xu, Y.; Kao, A.; Hsich, E.M.; Dew, M.A.; Kormos, R.L.; Pham, D.T.; Yancy, C.W.; LaRue, S.; et al. Improvement in cognitive function after heart transplant and mechanical circulatory support: Findings from the sustaining quality of life of the aged (sustain-it) study. Circulation 2019, 140.
- Bhat, G.; Yost, G.; Mahoney, E. Cognitive function and left ventricular assist device implantation. J. Hear. Lung Transplant. 2015, 34, 1398–1405.
- McIlvennan, C.; Bryce, K.; Lindenfeld, J.; Allen, L.; Lanfear, D. Assessment of Cognitive Function Prior to and After Implantation of Left Ventricular Assist Device. J. Hear. Lung Transplant. 2016, 35, S165–S166.
- Schall, R.R.; Petrucci, R.J.; Brozena, S.C.; Cavarocchi, N.C.; Jessup, M. Cognitive function in patients with symptomatic dilated cardiomyopathy before and after cardiac transplantation. J. Am. Coll. Cardiol. 1989, 14, 1666–1672.
- Georgiadis, D.; Sievert, M.; Cencetti, S.; Uhlmann, F.; Krivokuca, M.; Zierz, S.; Werdan, K. Cerebrovascular reactivity is im-paired in patients with cardiac failure. Eur. Heart J. 2000, 21, 407–413.
- Erkelens, C.D.; van der Wal, H.H.; de Jong, B.M.; Elting, J.-W.; Renken, R.; Gerritsen, M.; van Laar, P.J.; van Deursen, V.M.; van der Meer, P.; van Veldhuisen, D.J.; et al. Dynamics of cerebral blood flow in patients with mild non-ischaemic heart failure. Eur. J. Heart Fail. 2017, 19, 261–268.
- Fraser, K.S.; Heckman, G.A.; McKelvie, R.S.; Harkness, K.; Middleton, L.E.; Hughson, R.L. Cerebral hypoperfusion is exag-gerated with an upright posture in heart failure: Impact of depressed cardiac output. JACC Heart Fail. 2015, 3, 168–175.
- Bronzwaer, A.G.; Bogert, L.W.; Westerhof, B.E.; Piek, J.; Daemen, M.J.; Van Lieshout, J.J. Abnormal haemodynamic postural response in patients with chronic heart failure. ESC Hear. Fail. 2017, 4, 146–153.
- Kharraziha, I.; Holm, H.; Magnusson, M.; Wollmer, P.; Molvin, J.; Jujic, A.; Fedorowski, A.; Bachus, E.; Hamrefors, V. Impaired cerebral oxygenation in heart failure patients at rest and during head-up tilt testing. ESC Hear. Fail. 2020, 8, 586–594.
- Akiyama, H.; Barger, S.; Barnum, S.; Bradt, B.; Bauer, J.; Cole, G.M.; Cooper, N.R.; Eikelenboom, P.; Emmerling, M.; Fiebich, B.L.; et al. Inflammation and Alzheimer’s disease. Neurobiol. Aging 2000, 21, 383–421.
- Reichenberg, A.; Yirmiya, R.; Schuld, A.; Kraus, T.; Haack, M.; Morag, A.; Pollmächer, T. Cytokine-Associated Emotional and Cognitive Disturbances in Humans. Arch. Gen. Psychiatry 2001, 58, 445–452.
- Aukrust, P.; Ueland, T.; Lien, E.; Bendtzen, K.; Müller, F.; Andreassen, A.K.; Nordøy, I.; Aass, H.; Espevik, T.; Simonsen, S.; et al. Cytokine network in congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am. J. Cardiol. 1999, 83, 376–382.
- Torre-Amione, G.; Kapadia, S.; Benedict, C.; Oral, H.; Young, J.B.; Mann, D. Proinflammatory cytokine levels in patients with depressed left ventricular ejection fraction: A report from the studies of left ventricular dysfunction (SOLVD). J. Am. Coll. Cardiol. 1996, 27, 1201–1206.
- Willis, M.; Patterson, C. Proteotoxicity and Cardiac Dysfunction—Alzheimer’s Disease of the Heart? N. Engl. J. Med. 2013, 368, 455–464.
- Sweeney, P.; Park, H.; Baumann, M.; Dunlop, J.; Frydman, J.; Kopito, R.; McCampbell, A.; Leblanc, G.; Venkateswaran, A.; Nurmi, A.; et al. Protein misfolding in neurodegenerative diseases: Implications and strategies. Transl. Neurodegener. 2017, 6, 6.
- Kittleson, M.M.; Maurer, M.S.; Ambardekar, A.V.; Bullock-Palmer, R.P.; Chang, P.P.; Eisen, H.J.; Nair, A.P.; Nativi-Nicolau, J.; Ruberg, F.L.; and on behalf of the American Heart Association Heart Failure and Transplantation Committee of the Council on Clinical Cardiology. Cardiac Amyloidosis: Evolving Diagnosis and Management: A Scientific Statement from the American Heart Association. Circulation 2020, 142, e7–e22.
- Heling, A.; Zimmermann, R.; Kostin, S.; Maeno, Y.; Hein, S.; Devaux, B.; Bauer, E.; Klovekorn, W.P.; Schlepper, M.; Schaper, W.; et al. Increased expression of cytoskeletal, linkage, and extracellular proteins in failing human myocardium. Circ. Res. 2000, 86, 846–853.
- Cannon, J.A.; McMurray, J.J.; Quinn, T.J. ‘Hearts and minds’: Association, causation and implication of cognitive impairment in heart failure. Alzheimer’s Res. Ther. 2015, 7, 22.
- Kalantarian, S.; Stern, T.A.; Mansour, M.; Ruskin, J.N. Cognitive impairment associated with atrial fibrillation: A meta-analysis. Ann. Intern. Med. 2013, 158, 338–346.
- Alosco, M.L.; Spitznagel, M.B.; Sweet, L.H.; Josephson, R.; Hughes, J.; Gunstad, J. Atrial Fibrillation Exacerbates Cognitive Dysfunction and Cerebral Perfusion in Heart Failure. Pacing Clin. Electrophysiol. 2014, 38, 178–186.
- Kalaria, V.G.; Passannante, M.R.; Shah, T.; Modi, K.; Weisse, A.B. Effect of mitral regurgitation on left ventricular thrombus formation in dilated cardiomyopathy. Am. Hear. J. 1998, 135, 215–220.
- Freudenberger, R.S.; Hellkamp, A.S.; Halperin, J.L.; Poole, J.; Anderson, J.; Johnson, G.; Mark, D.B.; Lee, K.L.; Bardy, G.H. Risk of thromboembolism in heart failure: An analysis from the Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT). Circulation 2007, 115, 2637–2641.
