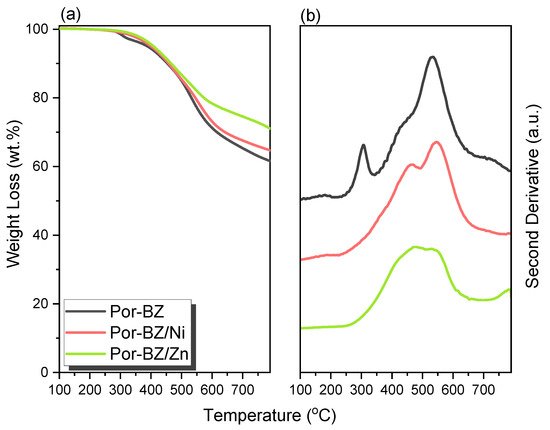
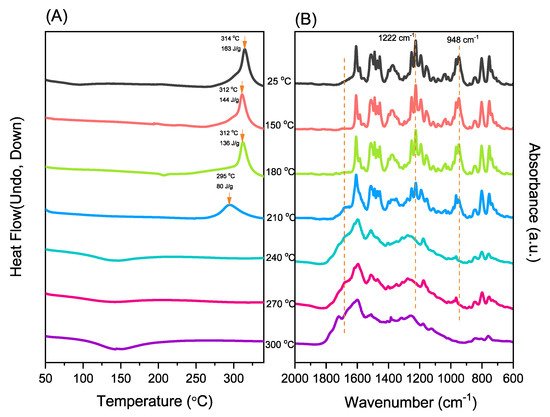
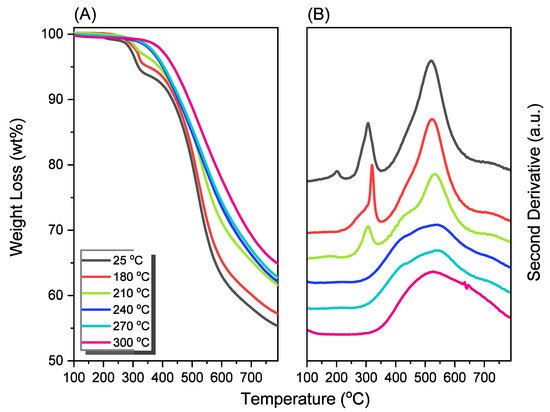
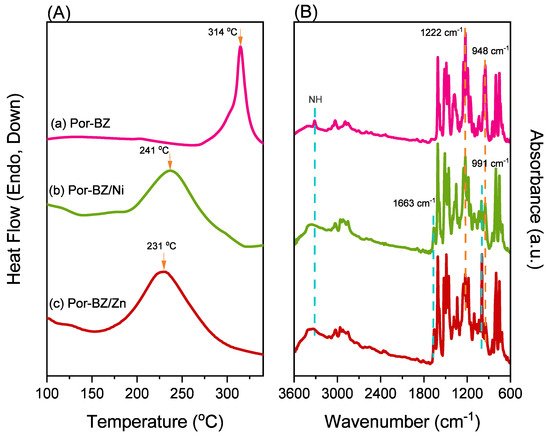
Porphyrin is a molecular material with many potential applications. New porphyrin-functionalized benzoxazine (Por-BZ) in high purity and yield was synthesized in this study based on 1H and 13C NMR and FTIR spectroscopic analyses through the reduction of Schiff base formed from tetrakis(4-aminophenyl)porphyrin (TAPP) and salicylaldehyde and the subsequent reaction with CH2O. Thermal properties of the product formed through ring-opening polymerization (ROP) of Por-BZ were measured using DSC, TGA and FTIR spectroscopy. Because of the rigid structure of the porphyrin moiety appended to the benzoxazine unit, the temperature required for ROP (314 °C) was higher than the typical Pa-type benzoxazine monomer (ca. 260 °C); furthermore, poly(Por-BZ) possessed a high thermal decomposition temperature (Td10 = 478 °C) and char yield (66 wt%) after thermal polymerization at 240 °C. An investigation of the thermal and luminescence properties of metal–porphyrin complexes revealed that the insertion of Ni and Zn ions decreased the thermal ROP temperatures of the Por-BZ/Ni and Por-BZ/Zn complexes significantly, to 241 and 231 °C, respectively. The metal ions acted as the effective promoter and catalyst for the thermal polymerization of the Por-BZ monomer, and also improved the thermal stabilities after thermal polymerization.
- polybenzoxazine
- ring-opening polymerization
- porphyrin
- metal complex
- thermal stability
1. Introduction
2. Metal Complexes of the Porphyrin-Functionalized Polybenzoxazine
2.1. Synthesis of TAPP
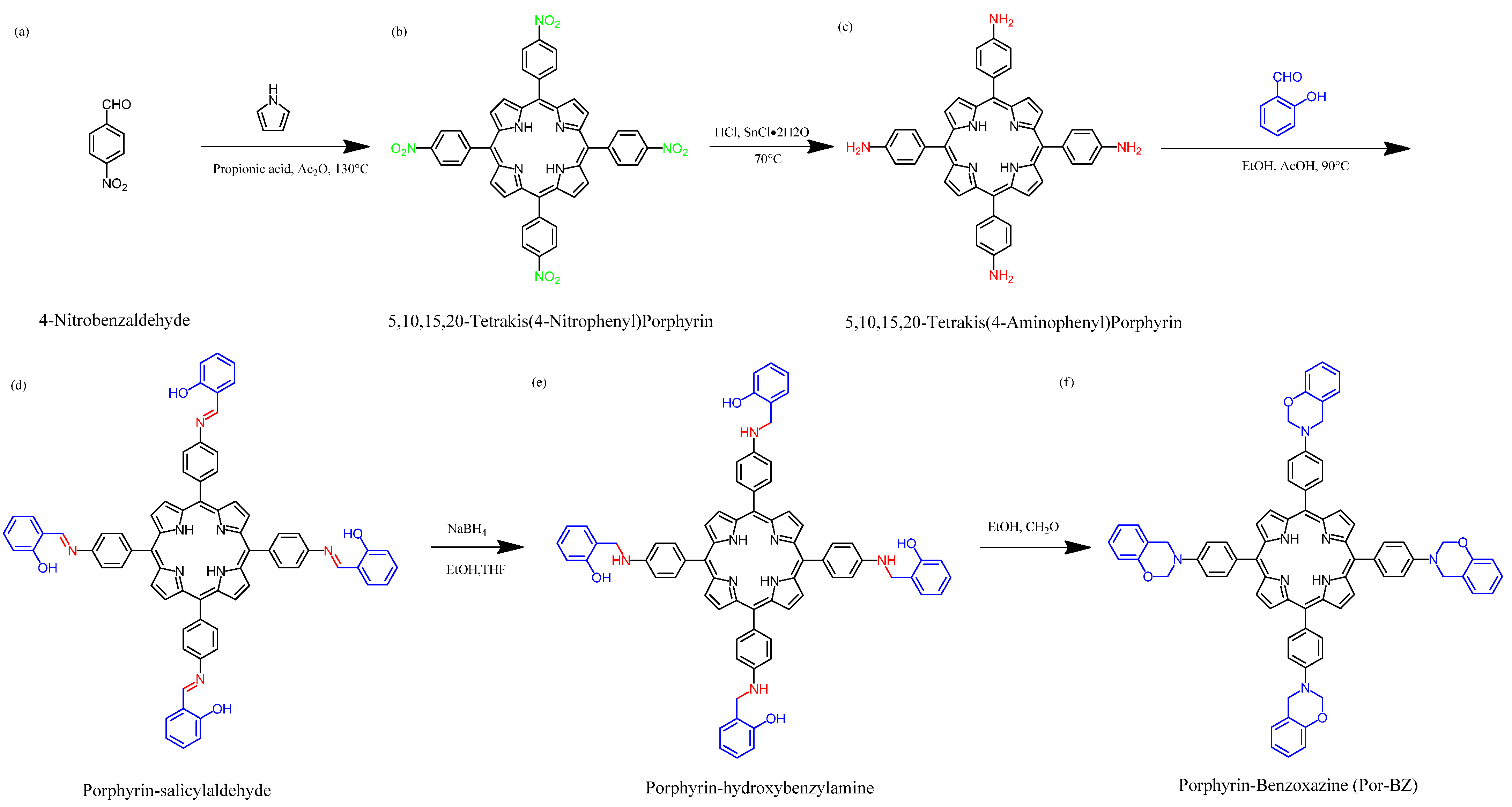
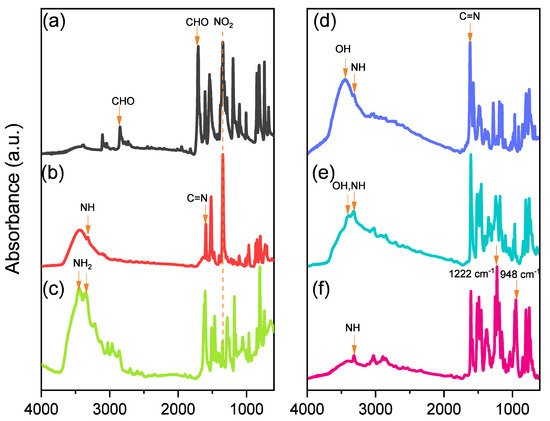
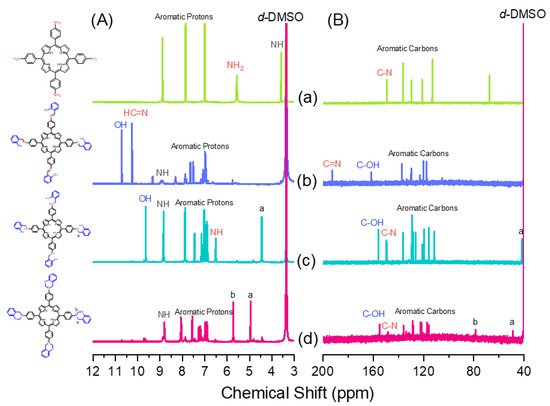
Scheme 1 presents the synthesis of the monomer Por-BZ from 4-nitrobenzaldehyde (Scheme 1a). First, the reseachers prepared TNPP (Scheme 1b) through the reaction of 4-nitrobenzaldehyde with pyrrole in the presence of propionic acid and acetic anhydride at 130 °C. The reduction of TNPP with HCl and SnCl2·H2O at 70 °C for 2 h provided TAPP as violet microcrystals in high yield and purity (Scheme 1c); FTIR and NMR spectroscopy confirmed the chemical structure.2.1. Synthesis of TAPP
2.2. Synthesis of Por-BZ
Although TAPP features four amino groups, one-pot Mannich condensations from primary amines, phenol, and CH2O do not always occur in the preparation of BZ monomers due to low selectivity, which is strongly dependent on the substituent positions. In previous studies [27], the reseachers used the approach described by Ishida and Lin et al. to prepare BZ rings through a three-step synthesis (Scheme 1d–f). Here, the reseachers used the Schiff base formed from TAPP and salicylaldehyde to first form porphyrin-salicylaldehyde (Por-Sa) (Scheme 1d). Next, the reseachers reduced Por-Sa to form the o-hydroxybenzylamine derivative Por-Hy (Scheme 1e). Finally, the reaction of Por-Hy with the aldehyde derivative was provided the target monomer Por-BZ (Scheme 1f).2.2. Synthesis of Por-BZ
2.3. Thermal Curing Polymerization of Por-BZ Monomer
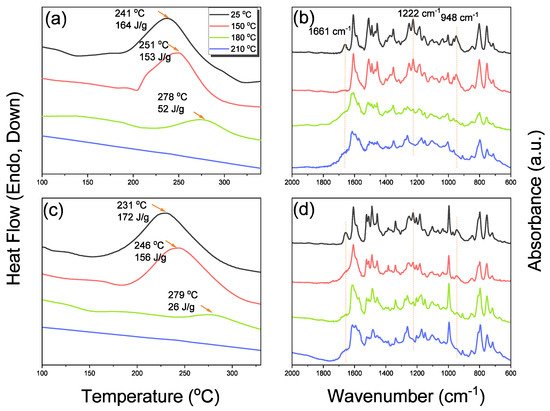
The reseachers used DSC, TGA, and FTIR spectroscopy to investigate the thermal curing polymerization of the Por-BZ monomer.2.3. Thermal Curing Polymerization of Por-BZ Monomer
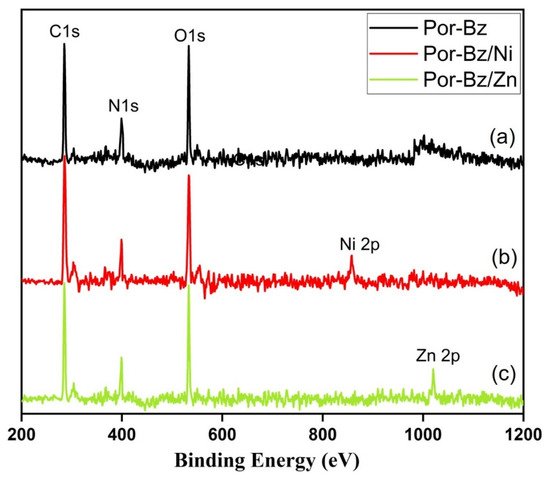
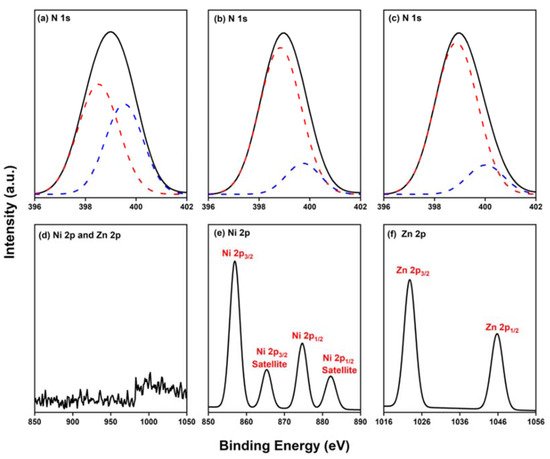
2.4. Characterization of Por-BZ/Metal Complex
Because porphyrins are excellent moieties for complexing metal cations, the reseachers tested the ability of the reearchers Por-BZ to bind Ni2.4. Characterization of Por-BZ/Metal Complex
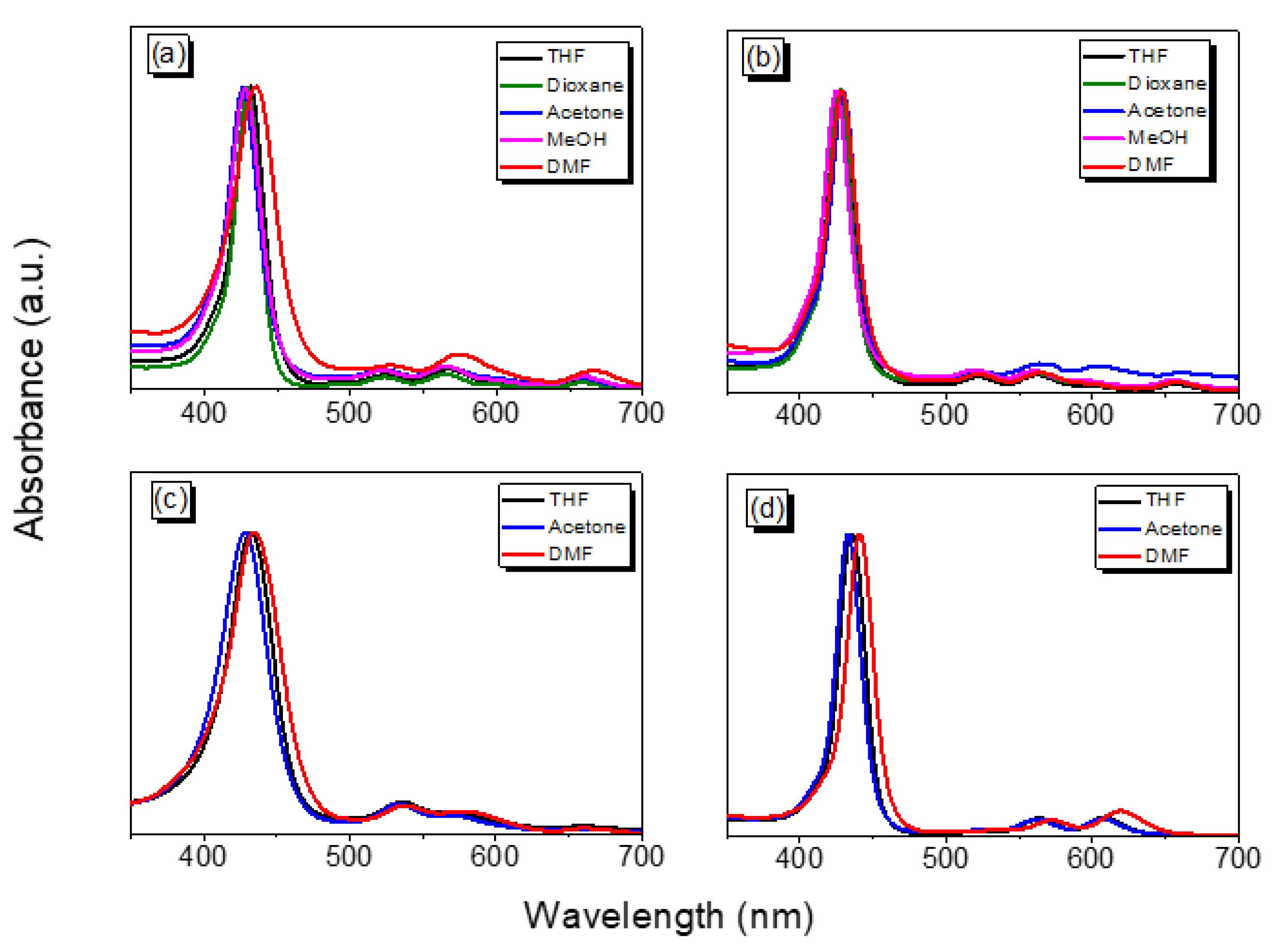
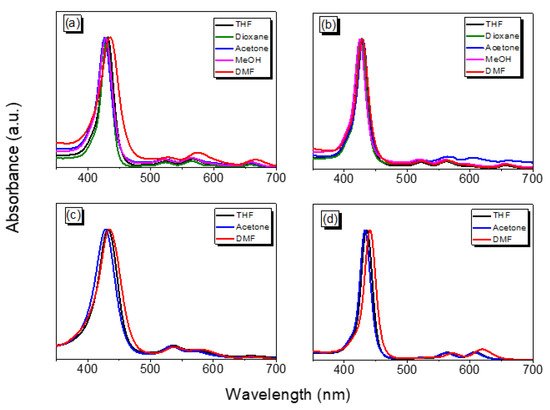
| Compound | Solvent | λabs (nm) (Soret Band) | λabs (nm) (Q Band) |
|---|---|---|---|
| TAPP | THF | 524, 566, 602, 662 | 432 |
| Dioxane | 524, 564, 600, 660 | 430 | |
| Acetone | 522, 564, 600, 660 | 428 | |
| MeOH | 522, 564, 596, 656 | 426 | |
| DMF | 528, 574, 608, 666 | 436 | |
| Por-BZ | THF | 522, 562, 598, 658 | 428 |
| Dioxane | 522, 562, 598, 656 | 428 | |
| Acetone | 520, 560, 596, 654 | 424 | |
| MeOH | 522, 564, 606, 662 | 430 | |
| DMF | 522, 564, 600, 658 | 428 | |
| Por-BZ/Ni | THF | 535, 574, 657.5 | 432 |
| Acetone | 533.5, 571, 656.5 | 428 | |
| DMF | 538, 579, 660.5 | 434 | |
| Por-BZ/Zn | THF | 564, 608 | 436 |
| Acetone | 562, 606 | 432 | |
| DMF | 572, 620 | 440 |
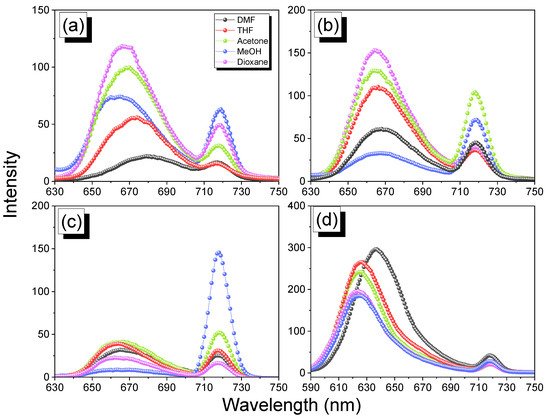
2.5. Thermal ROP of Por-BZ/Metal Complex
The reseachers used DSC analyses to monitor the thermal ROP behavior of Por-BZ/Ni and Por-BZ/Zn complexes after their thermal curing for 2 h at temperatures of 150, 180, and 210 °C, respectively.2.5. Thermal ROP of Por-BZ/Metal Complex