Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Dean Liu and Version 1 by Jayeeta Chattopadhyay.
In comparison to the undoped carbon nanomaterials, heteroatoms such as nitrogen-, sulphur-, boron-, phosphorous-, etc., incorporated nanomaterials have become well-accepted as potential electrocatalysts in water splitting, supercapacitors and dye-sensitized solar cells.
- carbon nanomaterials
- nitrogen doping
- sulphur doping
- co-doping
1. Nitrogen-Doped Metal-Free Carbon Nanostructured Electrocatalysts
1. Nitrogen-Doped Carbon Nanotube Electrocatalysts
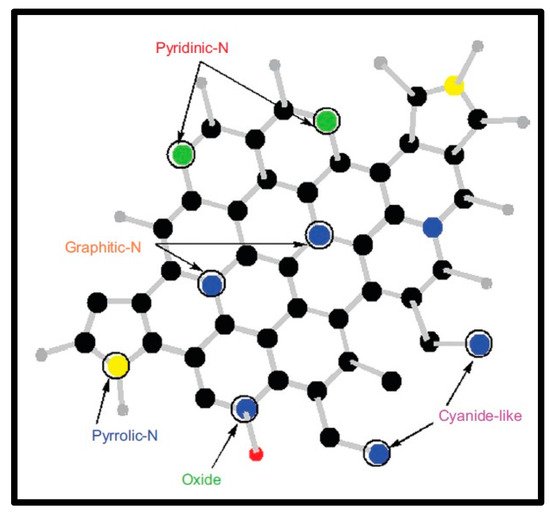
The functionalized nanotubes garnered significant attention in the field of the reinforced and conductive plastics, sensing materials and photovoltaic materials as scanning probe microscopy tips and many more applications. There have been two broad methods to synthesize substituted N-doped CNTs: (a) in situ process for insertion of nitrogen atom into the CNTs during the reaction, only [74,75,76,77,78][1][2][3][4][5]; and (b) postfunctionalization of CNTs with nitrogen by using various precursors and compounds like organic moieties. However, the postfunctionalization method has not been well investigated until now [79,80,81][6][7][8]. These nanomaterials have also been synthesized using other potential synthetic strategies, viz., arc discharge, laser ablation and plasma etching [82,83,84,85,86,87][9][10][11][12][13][14]. However, these methodologies required higher temperature conditions and a limited type of nitrogen or carbon precursors. Moreover, rapid evaporation of precursors and application of nitrogen or ammonia atmosphere has been required. In the chemical vapor deposition (CVD) method, the process could completed at a lower temperature range with and without the presence of an organometallic catalyst and by using a wide range of carbon or nitrogen precursors. This method could produce 20–25 g of N-carbon nanotubes per gram of catalyst, and nitrogen atoms are embedded into the hexagonal carbon network at various ratios with 10 atoms [81][8]. In the literature, nitrogen incorporation has been reported with nitrogen contents of <1 atom% to 20 atom% [75,88,89][2][15][16]. Highly oriented nanotubes with regular diameter and bond-length were termed in literature as “carpet-like” structures [90][17]. In this work, nitrogen was incorporated into the already synthesized CNT structure; however, this synthesis method was depicted as highly complex and tedious with multistep techniques. The first step is initiated with a chemical oxidation process of tips or structural defects of CNTs, followed by coupling with other molecules through carboxylic, carbonyl and/or hydroxyl groups. The covalent functionalization via bond formation to the π-conjugated structure of CNT leads to the rehybridization of sp2 bond to sp3. In this type of structure, nitrogen is attached to carbon following two different manners: (a) pyridine-type nitrogen, in which each nitrogen atom is bonded to two different carbon atoms, leading to the formation of cavities within the side wall of the tube, and (b) substitution N, in which a nitrogen atom bonds with three C atoms, as presented in Figure 21. Nitrogen contains an additional electron in its structure, in comparison to the carbon network; therefore, a nitrogen-incorporated CNT structure usually exhibits metallic properties [90,91,92][17][18][19]. The nitrogen group can also enhance the reactivity on the graphene in comparison to the pure CNT structures, which results in the potential applications of these materials in fast-responsive sensing technology; as effective field-emissions sources; and as polystyrene, epoxy composites, protein and nanoparticle immobilizers [78,93,94,95,96][5][20][21][22][23]. The most popular covalent functionality, with the application of plasma etching or by HNO3/H2SO4 treatment, includes carbonyl or carboxyl groups [97][24].
The plasma etching technique is essentially applicable during functionalization processes in a nitrogen atmosphere. In the next step, carboxyl groups are acylated with thionyl chloride to establish a basis for different amine compounds [98][25] or to combine with DNA and proteins [99,100][26][27]. The noncovalent functionalization is mostly conducted by adsorption or through the wrapping of the CNTs in polymer polynuclear aromatic compounds, surfactants or biomolecules by Vander Waals forces and π–π interactive forces. Other synthetic approaches of CNTs include the arc evaporation method of graphite [82,101][9][28]. The noncovalent methods are more favourable over covalent, as the chemical functionalization can be performed on the CNTs without affecting their structures and electronic networks on the nanotubular structures.
1.2. Chemical Vapour Deposition (CVD) Method
2. Chemical Vapour Deposition (CVD) Method
Chemical vapour deposition (CVD) is a technique to synthesize carbon nanotubes in bulk amounts, which involves the pyrolysis of different organic molecules, viz., CH4, C6H6, C2H2, etc., in inert atmosphere over Ni, Co, Fe, etc., catalysts [102,103][29][30]. Due to the simplicity and cost-effectiveness of CVD, researchers prefer to follow this methodology during the functionalization process.
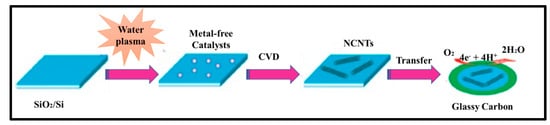
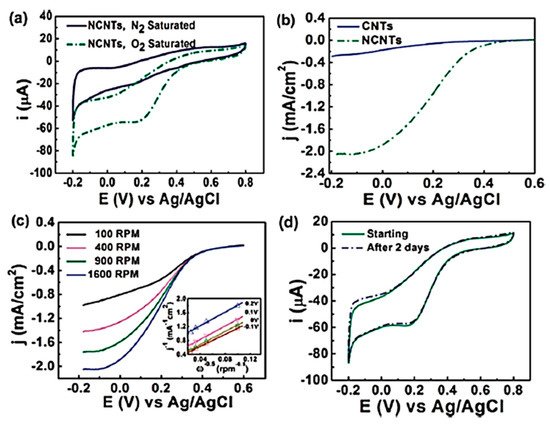
In 1997, Dai et al. introduced H2O plasma etching technology to generate surface patterns of polar groups with oxygen [104][31]. This methodology was further followed by Yu et al. [86][13] to develop SiO2 nanoparticles as the metal-free catalysts, in which a SiO2/Si wafer with a 30 nm-thick SiO2 coating was employed with H2O plasma etching at 30 W, 250 kHz and 0.62 Torr for 20 min. This plasma-etched substrate was placed into a tube furnace for the synthesis of CNTs by using the CVD method. Figure 32 represents the schematic diagram to represent the growth of CNTs. These materials acted as potential electrocatalysts in an oxygen reduction reaction analysed in 0.5 M H2SO4 solution saturated with N2 or O2. Figure 43 shows the various electrochemical studies conducted in this work. All the electrocatalytic studies have shown excellent results and long-term stability in an acidic medium in comparison to undoped CNTs. The authors also claimed that, due to the highly generic nature of the plasma etching technique, this synthetic strategy can be well accepted in various fields, from energy applications to electronic and biomedical systems [86][13]. Kim et al. mentioned a similar synthesis process in an Ar atmosphere at 800 °C for 1 h duration in which ferrocene, pyridine or ethylenediamine used as a catalyst carbon and nitrogen precursor, respectively [50][32]. TEM images of bamboo-structured N-doped CNTs (NCNTs) are presented in Figure 54. These products were used as excellent electrocatalysts in ORR of fuel cell applications. The same research group reported synthesis of nitrogen-doped CNTs by following a single step the CVD method in which either ferrocene or iron (II) phthalocyanine was used as the catalyst and pyridine as the carbon and nitrogen precursor, respectively. These materials have also used successfully as ORR electrocatalysts [105][33].
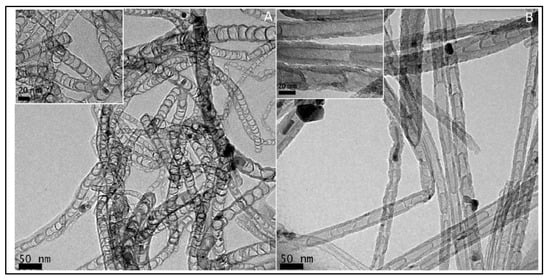
In 2011, Feng et al. [52][34] reported N-doped CNTs to be effective electrocatalysts in microbial fuel cells (MFCs), boasting cost-effectiveness and long durability. Moreover, these materials were depicted as more effective cathodic catalysts than the commonly used platinum catalyst with a maximum power density value of 1600 ± 50 mW·m−2. These N-doped CNTs have shown a lower drop in the percentage of power density than that of Pt/C over 25 cycles. Another research group reported the CVD synthesis floating catalyst method of nitrogen-doped carbon nanotubes using ferrocene/aniline together with toluene as an added carbon source [106][35]. Yang et al. synthesized aligned nitrogen-doped CNT bundles over 700–800 °C by taking ammonium-exchanged zeolite-β as the substrate material, ferric nitrate as the catalyst and acetonitrile as the carbon precursor [107][36]. In the same year, He et al. reported controllable synthesis of aligned CNx with a large surface area by pyrolyzing CH3CN/Fe(C5H5)2 on SiO2 and Si substrates over the temperature range of 750–900 °C. The specific diameters of CNTs diminished on Si substrates in comparison to a well-documented rise with temperature on silica, as the growth process followed different mechanisms of formation of catalyst particles [108][37]. Kim et al. developed N-doped, double-walled CNTs using chemical vapor deposition in which a CH4/NH3/Ar mixture flowed with the rate of 50/10/500 sccm on MgO-supported catalyst powders at the temperature of 850 °C for 10–30 min. of duration [109][38]. The synthesized CNTs are formed with a diameter of 10–20 nm. The SEM and HRTEM images of N-doped double walled-CNTs presented in Figure 65.
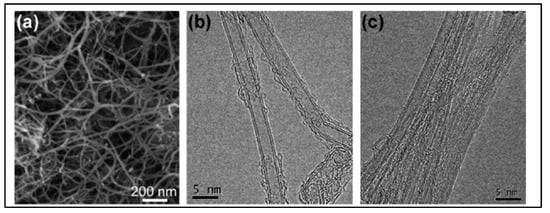
Figure 65. (a) SEM image shows the nanotube bundles with a diameter of 10–20 nm (b) HRTEM image reveals that the bundles are consisted mainly of double walled CNTs DWNTs. (c) A magnified image showing the outer diameter in the range of 1.5–2 nm (Reproduced with permission from Kim et al. [109][38]).
In recent years, Li et al. reported a one-step CVD method to synthesize three-dimensional nitrogen-doped CNT/graphene hybrid material on nickel foam [110][39]. In this study, nickel foam and melamine were mixed with the mass ratio of 1:5 kept in a horizontal quartz tube reactor and heated to within the temperature range of 600–800 °C in a hydrogen atmosphere for around 20 min at a flow rate of 70 sccm. In 2020, another research group mentioned a two-step synthetic strategy to develop nitrogen-doped carbon nanotubes derived from g-C3N4 [111][40]. In this case, exfoliated graphitic carbon nitride was functionalized with nickel oxides and placed in a ceramic boat to keep in the tubular furnace at 900 °C in a nitrogen atmosphere. Hydrogen was introduced for 3 h in the first step and ethylene for 10 min for the reduction process. The synthesis of N-doped MWCNTs with straight structure was reported by Xu et al. by using phthalocyanine derivatives [112][41] and the mixture of ethylene/hydrogen and ammonia at around 680 °C in a presence of alumina-supported iron catalysts in a CVD furnace [113][42]. The amount of nitrogen incorporated into CNT can be controlled by using different amounts of nitrogen precursors [80,114][7][43]. The rate in which nanotubes grow during synthesis can be enhanced with the increase in its precursor significantly, resulting into the increase in intensity ratio of the D to G bands in Raman spectra. The inner structure of N-doped CNTs constitutes regular morphological transformation from the straight and smoother walls (0 atom% N) to 1.5 atom% N-containing, bamboo-structured CNTs; further, it changed to corrugated structures with 3.1 atom% and higher nitrogen [115][44]. It has been analysed by Wang et al. that, during the synthesis of N-doped CNTs, when melamine is used as the C/N initiator, it can incorporate 20 atom% nitrogen. In this type of synthesis method, the N atom present in the reaction medium self-assembled with gaseous carbon without any assistance from metal [116][45]. These N-doped CNTs were utilized successfully as ORR electrocatalysts in methanol fuel cells measured in alkaline media. Figure 76 shown the various electrochemical studies of NCNT/GC.
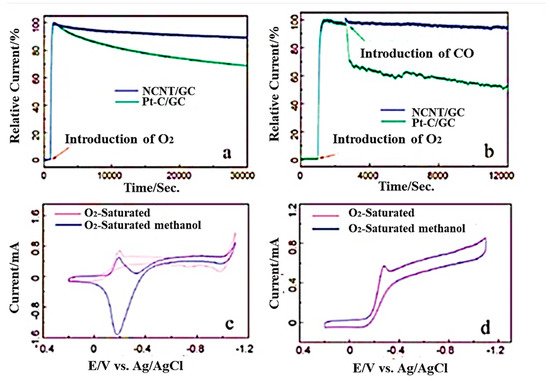
Figure 76. (a) Chronoamperometric studies with NCNT/GC and Pt–C/GC electrodes in oxygen saturated 0.1M KOH. (b) Responses after the introduction of 10% CO. (c) Cyclic voltammograms of Pt/GC and (d) cyclic voltammograms of NCNT/GC electrodes oxygen saturated 0.1 M KOH, with and without 3 M methanol solution (Reproduced with permission from Wang et al. [116][45]).
The incorporation of nitrogen atoms usually shows very strong ability to promote the self-assembled CNTs. Nitrogen could create highly active sites in carbon networks, which results in remarkable electrocatalytic performance comparable to traditional Pt-based materials as electrocatalysts. Their high activity with excellent stability and selectivity always made N-doped CNTs better electrocatalysts in this purpose. These materials were also strongly resistant to CO poisoning, had a robust structure and were economically favourable. Due to doping in CNT structures, a basic shape could be transformed from a hollow cylinder to a bamboo-shaped structure. The resultant doped materials contained plenty of compartments, the lengths of which gradually decreased with variation in N concentration [117][46].
1.3. Chemical and Electrochemical Modification Method
3. Chemical and Electrochemical Modification Method
The chemical modification methods to synthesize nitrogen-doped CNTs included two different approaches, viz., covalent and noncovalent. During the covalent modification, oxygen-containing functional groups, viz., carboxyl and hydroxyl, were formed and generated on the surface. Among the functional groups, carboxylic acid groups were chosen as the best options, as they could easily proceed a variety of reactions in the modification process and could be easily developed using different oxidizing treatments, e.g., ozonolysis, sonication in nitric and sulfuric acid, refluxing in nitric acid, etc. In the next step, carboxyl-functionalized CNTs were grafted with the functional moieties by using the terminal oxidation process following various mechanisms from the defect site, from chemistry oxidation reactions and esterification/amidation processes to the already oxidized CNTs [118[47][48],119], mechanochemical modification [120[49][50],121], ionic liquids, cycloaddition reactions [122[51][52],123], electrochemical modification reactions, diazotization [124][53] and radical additions [125][54].
The efficient and successful doping and tailoring technologies in CNTs involved the controlling of redox properties of the dopant. Nitrogen-doped CNTs have excellent electrocatalytic activity compared to Pt electrodes, which could be acclaimed by the formation of additional active sites on the surface of the materials and has led to better dispersion of the Pt particles over the N–CNT and performed better in methanol oxidation [126][55]. From the results, it was analysed that doped CNTs as electrode materials always enhanced the output power of the thermoelectrochemical cells. Doping enhanced the electrochemical active surface area (ESCA) values in the CNT electrodes in proportional way. Wei et al. reported doped CNTs mixed with glutaraldehyde functionalized chitosan (GCS), which depicted an improved biocompatibility and higher conductivity in enzyme immobilization process, due to the enhanced kinetics from the N–CNTs [127][56]. The electrochemical modification process was carried out through two types of coupling reactions, working under oxidative or reductive conditions. In 2002, Kooi et al. worked on anodic coupling reaction to the SWCNTs by using two different aromatic amines, viz., 4-aminobenzylamine and 4-aminobenzoic acid [128][57]. The noncovalent functionalization process could be carried out through the porphyrin assembled on the N-doped MWCNTs via the Fe-N coordination. Tu et al. reported this noncovalent modification by porphyrin, which led the MWNTs insoluble in water, however, performed well as catalysts and biosensors [129][58].
1.4. Nitrogen-Doped Carbon Hollow Spheres
4. Nitrogen-Doped Carbon Hollow Spheres
The carbon spheres usually referred to the spherical shaped carbon in semicrystalline or crystalline form, and constituted solid, hollow or core-shell morphological structures. Researchers have paid huge attention to nitrogen-doped hollow spherical structures in recent years due to their lower density, greater surface area values, better electrical conductivity, and excellent structural stability. In 2012, Zhu et al. developed a hierarchical porous hollow carbon nanospheres as an oxygen reduction electrocatalyst for zinc–air batteries, which contained active pyridinic-N and graphitic-N by using polystyrene spheres and aniline as the corresponding template and precursor [130][59]. Gu et al. reported N-doped porous carbon spheres with excellent porosity characteristics, which was used as a potential electrocatalyst in ORR. The unique spherical structures with remarkable stability and recyclability made these materials the most promising ORR electrocatalysts [131][60]. Hydrothermal carbonization method was adopted to make these materials by using biomass glucose, followed by treatment in ammonia and by subsequent activation treatment. Another research group reported the development of N-doped carbon nanodots @ nanospheres, which were applied as efficient electrocatalyst in ORR, in which high electrocatalytic activity was shown with an onset potential of −0.08 V, and they showed greater durability and greater resistance to the methanol cross-over effect; these results were comparable to commercially available Pt/C electrocatalyst. These N-doped carbon nanodots of sizes 2–6 nm were successfully formed by using the hydrothermal method from natural biomass (e.g., fresh grass) at 180 °C for 10 h duration. Furthermore, these carbon nanodots were subsequently immobilized onto functionalized microporous carbon nanospheres (MCNSs) with an average diameter of ∼100 nm and a surface area of 241 m2 g−1 via a simple hydrothermal process to self-assemble a carbon-based nanocomposite (N-CNDs@MCNSs) in the presence of oxygen (O)-containing surface functional groups [132][61]. Today, a significant number of research works were carried out on nitrogen encapsulation on metal/metal oxides/carbon nanosphere materials potentially applied as electrodes or electrocatalysts [133,134,135,136,137,138,139,140][62][63][64][65][66][67][68][69].
1.5. Nitrogen-Doped Graphene Electrocatalysts
5. Nitrogen-Doped Graphene Electrocatalysts
Graphene has been two-dimensionally structured with sp2 hybridized carbon with interesting physical and chemical characteristics. To achieve the desired performance in electrochemical and biochemical applications, nitrogen-enriched graphene materials were synthesized using a wide range of methodologies [141,142,143,144,145,146,147,148,149,150,151][70][71][72][73][74][75][76][77][78][79][80]. In 2011, Zhang et al. developed N-doped graphene by thermally annealing graphene oxide in the presence ammonia [152][81]. Another research group reported a facile and catalyst-free method to develop large-scale synthesis of nitrogen-doped graphene with 10.1 wt% nitrogen content by using the economically favourable industrial material melamine as the nitrogen source [153][82]. Sheng et al. synthesized nitrogen-doped graphene using the solvothermal method with the reaction between tetrachloromethane with lithium nitride under mild conditions [154][83]. Figure 86 and Figure 97 present the schematic diagram to synthesize these materials and their potential electrocatalytic applications in ORR under alkaline media, respectively. Another simple way to produce N-doped graphene nanosheets following the solvothermal route is via the reaction between graphene oxide and urea with a nitrogen content of 10.13 atom% [155][84]. Temperature played a pivotal role in the solvothermal process during the doping of nitrogen in the graphene network [152,153][81][82]. Another research group developed pyrrolic and pyridinic type nitrogen incorporation in a graphene structure at 300 and 500 °C, respectively, with the annealing treatment of graphene oxide in the presence of glycine and AgNO3 [156][85]. This particular methodology produced N-doped graphene with 13.5 atom% of nitrogen into the materials. The CVD method was also adopted by using methane and ammonia; these materials were utilized as metal-free electrocatalysts in ORR applied in fuel cells [157][86]. Many research groups have also applied the arc discharge method in H2 and He atmosphere and under pyridine vapour to produce the nitrogen-doped graphene structure [158,159][87][88].
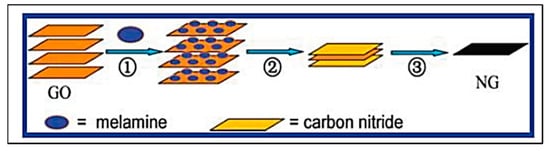
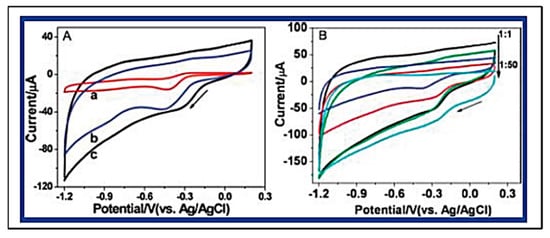
Figure 97. (A) Typical cyclic voltammograms (CVs) for ORR obtained at a bare GCE (a), graphene/GCE (b), and NG5/GCE (N% = 7.1%) (c) in O2 saturated 0.1 M KOH aqueous solution. (B) CVs for ORR at NGs, synthesized with different mass ratio of GO and melamine (1:1, 1:2, 1:5, 1:10, 1:50) at 800 °C, modified GCE in O2 saturated 0.1 M KOH aqueous solution. (Reproduced with permission from Sheng et al. [154][83]).
Yang et al. reported synthesis of N-doped graphene, the result of which was successfully demonstrated as highly efficient metal-free bifunctional electrocatalysts in the oxygen reduction and evolution reaction [160][89]. In this report, e- donating quaternary nitrogen sites were responsible for ORR; in contrast, e- withdrawing pyridinic nitrogen acted as active sites in OER, resulting into greater transports of electrons and electrolytes [160][89]. The schematic diagram to synthesize N-doped graphene nanoribbon networks shown in Figure 10 [160][89].
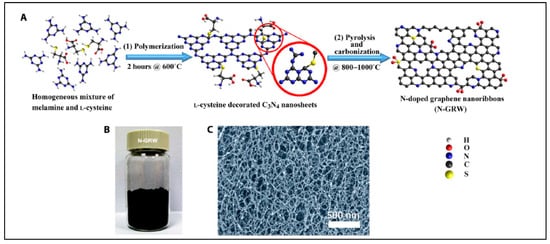
Figure 10. Synthesis of N-doped graphene nanoribbon networks (GRW). (A) Synthesis steps: (1) polymerization at 600 °C for 2 h, and (2) pyrolysis and carbonization at 800° to 1000 °C. (B) Digital photograph of the as-synthesized N-GRW. (C) Scanning electron microscopy (SEM) image of the as-synthesized N-GRW (Reproduced with permission from Yang et al. [160][89]).
Liu et al. synthesized pyrrolic–nitrogen doped graphene, which was successfully adapted as carbon-free electrocatalysts in electrocatalytic reduction of carbon dioxide to formic acid in their comparative study with the computational method [161][90]. Earlier, Ju et al. developed nitrogen-doped graphene nanoplatelets as potential metal-free, counter-electrode materials used in organic dye-sensitized solar cells [162][91]. Rahsepar et al. followed a hybrid hydrothermal-microwave process to synthesize N-doped graphene, which exhibited remarkable electrocatalytic activity in ORR [163][92]. The number of catalytic sites was enhanced due to the incorporation of N-atom into graphene. Maouche et al. developed nitrogen-doped graphene with porous structures, which was successfully employed as an ORR electrocatalyst [164][93]. In this work, a facile fabrication technology was carried out with graphitic carbon nitride (g-C3N4) and graphene oxide (GO) as raw materials. Another research group utilized N-doped graphene as an electrocatalyst in ORR under alkaline medium and in anion exchange membrane fuel cells [165][94].
References
- Choi, H.C.; Park, J.; Kim, B. Distribution and Structure of N Atoms in Multiwalled Carbon Nanotubes Using Variable-Energy X-Ray Photoelectron Spectroscopy. J. Phys. Chem. B 2005, 109, 4333–4340.
- Hu, J.; Yang, P.; Lieber, C.M. Nitrogen-driven sp3 to sp2 transformation in carbon nitride materials. Phys. Rev. B 1998, 57, R3185.
- Ayala, P.; Arenal, R.; Rümmeli, M.H.; Rubio, A.; Pichler, T. The doping of carbon nanotubes with nitrogen and their potential applications. Carbon 2010, 48, 575–586.
- Yang, J.; Liu, D.-J.; Kariuki, N.N.; Chen, L.X. Aligned carbon nanotubes with built-in FeN4 active sites for electrocatalytic reduction of oxygen. Chem. Commun. 2008, 3, 329–331.
- Lee, D.H.; Lee, W.J.; Kim, S.O. Highly Efficient Vertical Growth of Wall-Number-Selected, N-Doped Carbon Nanotube Arrays. Nano Lett. 2009, 9, 1427–1432.
- Ramanathan, T.; Fisher, F.T.; Ruoff, R.S.; Brinson, L.C. Amino-Functionalized Carbon Nanotubes for Binding to Polymers and Biological Systems. Chem. Mater. 2005, 17, 1290–1295.
- Li, J.; Vergne, M.J.; Mowles, E.D.; Zhong, W.-H.; Hercules, D.M.; Lukehart, C.M. Surface functionalization and characterization of graphitic carbon nanofibers (GCNFs). Carbon 2005, 43, 2883–2893.
- Tang, C.; Bando, Y.; Golberg, D.; Xu, F. Structure and nitrogen incorporation of carbon nanotubes synthesized by catalytic pyrolysis of dimethylformamide. Carbon 2004, 42, 2625–2633.
- Stephan, O.; Ajayan, P.M.; Colliex, C.; Redlich, P.; Lambert, J.M.; Bernier, P.; Lefin, P. Doping Graphitic and Carbon Nanotube Structures with Boron and Nitrogen. Science 1994, 266, 1683–1685.
- Droppa, R., Jr.; Hammer, P.; Carvalho, A.C.M.; dos Santos, M.C.; Alvarez, F. Incorporation of nitrogen in carbon nanotubes. J. Non-Cryst. Solids 2002, 299–302, 874–879.
- Hu, J.; Yang, P.; Lieber, C.M. Nitrogen driven structural transformation in carbon nitride materials. Appl. Surf. Sci. 1998, 127–129, 569–573.
- Rodil, S.E.; Milne, W.I.; Robertson, J.; Brown, L.M. Maximized sp3 bonding in carbon nitride phases. Appl. Phys. Lett. 2000, 77, 1458–1460.
- Yu, D.; Zhang, Q.; Dai, L. Highly Efficient Metal-Free Growth of Nitrogen-Doped Single-Walled Carbon Nanotubes on Plasma-Etched Substrates for Oxygen Reduction. J. Am. Chem. Soc. 2010, 132, 15127–15129.
- Luais, E.; Thobie-Gautier, C.; Tailleur, A.; Djouadi, M.-A.; Granier, A.; Tessier, P.Y.; Debarnot, D.; Poncin-Epaillard, F.; Boujtita, M. Preparation and modification of carbon nanotubes electrodes by cold plasmas processes toward the preparation of amperometric biosensors. Electrochim. Acta 2010, 55, 7916–7922.
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58.
- Majeed, S.; Zhao, J.; Zhang, L.; Anjum, S.; Liu, Z.; Xu, G. Synthesis and electrochemical applications of nitrogen-doped carbon nanomaterials. Nanotechnol. Rev. 2013, 2, 615–635.
- Ning, X.; Li, Y.; Dong, B.; Wang, H.; Yu, H.; Peng, F.; Yang, Y. Electron transfer dependent catalysis of Pt on N-doped carbon nanotubes: Effects of synthesis method on metal-support interaction. J. Catalysis. 2017, 348, 100–109.
- Terrones, M.; Grobert, N.; Terrones, H. Synthetic routes to nanoscale BxCyNz architectures. Carbon 2002, 40, 1665–1684.
- Ewels, C.P.; Glerup, M. Nitrogen Doping in Carbon Nanotubes. J. Nanosci. Nanotechnol. 2005, 5, 1345–1363.
- Villalpando-Páez, F.; Romero, A.H.; Muñoz-Sandoval, E.; Martínez, L.M.; Terrones, H.; Terrones, M. Fabrication of vapor and gas sensors using films of aligned CNx nanotubes. Chem. Phys. Lett. 2004, 386, 137–143.
- Doytcheva, M.; Kaiser, M.; Verheijen, M.A.; Reyes-Reyes, M.; Terrones, M.; de Jonge, N. Electron emission from individual nitrogen-doped multi-walled carbon nanotubes. Chem. Phys. Lett. 2004, 396, 126–130.
- Zhang, J.; Cheng, F.-F.; Zheng, T.-T.; Zhu, J.-J. Design and Implementation of Electrochemical Cytosensor for Evaluation of Cell Surface Carbohydrate and Glycoprotein. Anal. Chem. 2010, 82, 3547–3555.
- Souza, A.M.; Rocha, A.R.; Fazzio, A.; da Silva, A.J.R. Ab-initio calculations for a realistic sensor: A study of CO sensors based on nitrogen-rich carbon nanotubes. AIP Adv. 2012, 2, 032115.
- Terrones, M.; Kamalakaran, R.; Seeger, T.; Rühle, M. Novel nanoscale gas containers: Encapsulation of N2 in CNx nanotubes. Chem. Commun. 2000, 23, 2335–2336.
- Peng, Q.; He, X.; Li, Y.; Wang, C.; Wang, R.; Hu, P.; Yan, Y.; Sritharan, T. Chemically and uniformly grafting carbon nanotubes onto carbon fibers by poly(amidoamine) for enhancing interfacial strength in carbon fiber composites. J. Mater. Chem. 2012, 22, 5928–5931.
- Jiang, K.; Eitan, A.; Schadler, L.S.; Ajayan, P.M.; Siegel, R.W.; Grobert, N.; Mayne, M.; Reyes-Reyes, M.; Terrones, H.; Terrones, M. Selective Attachment of Gold Nanoparticles to Nitrogen-Doped Carbon Nanotubes. Nano Lett. 2003, 3, 275–277.
- Kim, K.K.; Kim, D.; Kim, S.K.; Park, S.M.; Song, J.K. Formation of ZnO nanoparticles by laser ablation in neat water. Chem. Phys. Lett. 2011, 511, 116–120.
- Iijima, S.; Ichihashi, T. Single-shell carbon nanotubes of 1-nm diameter. Nature 1993, 363, 603–605.
- Takenobu, T.; Takano, T.; Shiraishi, M.; Murakami, Y.; Ata, M.; Kataura, H.; Achiba, Y.; Iwasa, Y. Stable and controlled amphoteric doping by encapsulation of organic molecules inside carbon nanotubes. Nat. Mater. 2003, 2, 683–688.
- Terrones, M. Carbon nanotubes: Synthesis and properties, electronic devices and other emerging applications. Int. Mater. Rev. 2004, 49, 325–377.
- Dai, L.; Griesser, H.J.; Mau, A.W.H. Surface Modification by Plasma Etching and Plasma Patterning. J. Phys. Chem. B 1997, 101, 9548–9554.
- Chen, Z.; Higgins, D.; Tao, H.; Hsu, R.S.; Chen, Z. Highly Active Nitrogen-Doped Carbon Nanotubes for Oxygen Reduction Reaction in Fuel Cell Applications. J. Phys. Chem. C 2009, 113, 21008–21013.
- Chen, Z.; Higgins, D.; Chen, Z. Electrocatalytic activity of nitrogen doped carbon nanotubes with different morphologies for oxygen reduction reaction. Electrochim. Acta 2010, 55, 4799–4804.
- Feng, L.; Yan, Y.; Chen, Y.; Wang, L. Nitrogen-doped carbon nanotubes as efficient and durable metal-free cathodic catalysts for oxygen reduction in microbial fuel cells. Energy Environ. Sci. 2011, 4, 1892–1899.
- Nxumalo, E.N.; Nyamori, V.O.; Coville, N.J. CVD synthesis of nitrogen doped carbon nanotubes using ferrocene/aniline mixtures. J. Organomet. Chem. 2008, 693, 2942–2948.
- Yang, Z.; Xia, Y.; Mokaya, R. Aligned N-Doped Carbon Nanotube Bundles Prepared via CVD Using Zeolite Substrates. Chem. Mater. 2005, 17, 4502–4508.
- He, M.; Zhou, S.; Zhang, J.; Liu, Z.; Robinson, C. CVD Growth of N-Doped Carbon Nanotubes on Silicon Substrates and Its Mechanism. J. Phys. Chem. B 2005, 109, 9275–9279.
- Kim, S.Y.; Lee, J.; Na, C.W.; Park, J.; Seo, K.; Kim, B. N-doped double-walled carbon nanotubes synthesized by chemical vapor deposition. Chem. Phys. Lett. 2005, 413, 300–305.
- Li, H.-F.; Wu, F.; Wang, C.; Zhang, P.-X.; Hu, H.-Y.; Xie, N.; Pan, M.; Zeng, Z.; Deng, S.; Wu, M.H.; et al. One-Step Chemical Vapor Deposition Synthesis of 3D N-doped Carbon Nanotube/N-doped Graphene Hybrid Material on Nickel Foam. Nanomaterials 2018, 8, 700.
- Maślana, K.; Kaleńczuk, R.J.; Zielińska, B.; Mijowska, E. Synthesis and Characterization of Nitrogen-doped Carbon Nanotubes Derived from g-C3N4. Materials 2020, 13, 1349.
- Xu, Z.; Li, H.; Fu, M.; Luo, H.; Sun, H.; Zhang, L.; Li, K.; Wei, B.; Lu, J.; Zhao, X. Nitrogen-doped carbon nanotubes synthesized by pyrolysis of nitrogen-rich metal phthalocyanine derivatives for oxygen reduction. J. Mater. Chem. 2012, 22, 18230–18236.
- Gulino, G.; Vieira, R.; Amadou, J.; Nguyen, P.; Ledoux, M.J.; Galvagno, S.; Centi, G.; Cuong, P.H. Synthesis of carbon nanotubes by chemical vapour deposition. Appl. Catal. A Gen. 2005, 279, 89–97.
- Dong, J.; Qu, X.; Wang, L.; Zhao, C.; Xu, J. Electrochemistry of Nitrogen-Doped Carbon Nanotubes (CNx) with Different Nitrogen Content and Its Application in Simultaneous Determination of Dihydroxybenzene Isomers. Electroanalysis 2008, 20, 1981–1986.
- Liu, H.; Zhang, Y.; Li, R.; Sun, X.; Désilets, S.; Abou-Rachid, H.; Jaidann, M.; Lussier, L.-S. Structural and morphological control of aligned nitrogen-doped carbon nanotubes. Carbon 2010, 48, 1498–1507.
- Wang, Z.; Jia, R.; Zheng, J.; Zhao, J.; Li, L.; Song, J.; Zhu, Z. Nitrogen-Promoted Self-Assembly of N-Doped Carbon Nanotubes and Their Intrinsic Catalysis for Oxygen Reduction in Fuel Cells. ACS Nano 2011, 5, 1677–1684.
- Xu, E.; Wei, J.; Wang, K.; Li, Z.; Gui, X.; Jia, Y.; Zhu, H.; Wu, D. Doped carbon nanotube array with a gradient of nitrogen concentration. Carbon 2010, 48, 3097–3102.
- Abuilaiwi, F.A.; Laoui, T.; Al-Harthi, M.; Atieh, M.A. Modification and functionalization of multiwalled carbon nanotube (MWCNT) via Fischer esterification. Arab. J. Sci. Eng. 2010, 35, 37–48.
- Jiang, K.; Schadler, L.S.; Siegel, R.W.; Zhang, X.; Zhang, H.; Terrones, M. Protein immobilization on carbon nanotubes via a two-step process of diimide-activated amidation. J. Mater. Chem. 2004, 14, 37–39.
- Ambrogi, V.; Gentile, G.; Ducati, C.; Oliva, M.C.; Carfagna, C. Multiwalled carbon nanotubes functionalized with maleated poly(propylene) by a dry mechano-chemical process. Polymer 2012, 53, 291–299.
- Chen, L.; Xie, H.; Li, Y.; Yu, W. Surface Chemical Modification of Multiwalled Carbon Nanotubes by a Wet-Mechanochemical Reaction. J. Nanomater. 2008, 2008, 783981.
- Fechler, N.; Fellinger, T.-P.; Antonietti, M. Template-Free One-Pot Synthesis of Porous Binary and Ternary Metal Carbon Composites from Ionic Liquids. Chem. Mater. 2012, 24, 713–719.
- Pan, C.; Qiu, L.; Peng, Y.; Yan, F. Facile synthesis of nitrogen-doped carbon–Pt nanoparticle hybrids via carbonization of poly(-co-acrylonitrile) for electrocatalytic oxidation of methanol. J. Mater. Chem. 2012, 22, 13578–13584.
- Fan, L.; Chen, J.; Zhu, S.; Wang, M.; Xu, G. Determination of Cd2+ and Pb2+ on glassy carbon electrode modified by electrochemical reduction of aromatic diazonium salts. Electrochem. Commun. 2009, 11, 1823–1825.
- Wu, H.-X.; Tong, R.; Qiu, X.-Q.; Yang, H.-F.; Lin, Y.-H.; Cai, R.-F.; Qian, S.-X. Functionalization of multiwalled carbon nanotubes with polystyrene under atom transfer radical polymerization conditions. Carbon 2007, 45, 152–159.
- Maiyalagan, T. Synthesis and electro-catalytic activity of methanol oxidation on nitrogen containing carbon nanotubes supported Pt electrodes. Appl. Catal. B: Environ. 2008, 80, 286–295.
- Wei, W.; Li, P.; Li, Y.; Cao, X.; Liu, S. Nitrogen-doped carbon nanotubes enhanced laccase enzymatic reactivity towards oxygen reduction and its application in biofuel cell. Electrochem. Commun. 2012, 22, 181–184.
- Kooi, S.E.; Schlecht, U.; Burghard, M.; Kern, K. Electrochemical Modification of Single Carbon Nanotubes. Angew. Chem. Int. Ed. 2002, 41, 1353–1355.
- Tu, W.; Lei, J.; Jian, G.; Hu, Z.; Ju, H. Noncovalent Assembly of Picket-Fence Porphyrins on Nitrogen-Doped Carbon Nanotubes for Highly Efficient Catalysis and Biosensing. Chemistry 2010, 16, 4120–4126.
- Zhu, J.; Zhou, H.; Zhang, C.; Zhang, J.; Mu, S. Dual active nitrogen doped hierarchical porous hollow carbon nanospheres as an oxygen reduction electrocatalyst for zinc–air batteries. Nanoscale 2017, 9, 13257–13263.
- Gu, D.; Ma, R.; Zhou, Y.; Wang, F.; Yan, K.; Liu, Q.; Wang, J. Synthesis of Nitrogen-Doped Porous Carbon Spheres with Improved Porosity toward the Electrocatalytic Oxygen Reduction. ACS Sustain. Chem. Eng. 2017, 5, 11105–11116.
- Zhang, H.; Chen, J.; Li, Y.; Liu, P.; Wang, Y.; An, T.; Zhao, H. Nitrogen-Doped Carbon as An Efficient Electrocatalyst for Oxygen Reduction Reaction. Electrochim. Acta 2015, 165, 7–13.
- Wu, L.; Shen, Y.; Yu, L.; Xi, J.; Qiu, X. Boosting vanadium flow battery performance by Nitrogen-doped carbon nanospheres electrocatalyst. Nano Energy 2016, 28, 19–28.
- Wang, M.; Peng, F.; Wang, M.; Han, J. N-Doped carbon nanospheres with nanocavities to encapsulate manganese oxides as ORR electrocatalysts. New J. Chem. 2020, 44, 14915–14921.
- Wu, X.; Niu, Y.; Feng, B.; Yu, Y.; Huang, X.; Zhong, C.; Hu, W.; Li, C.M. Mesoporous Hollow Nitrogen-Doped Carbon Nanospheres with Embedded MnFe2O4/Fe Hybrid Nanoparticles as Efficient Bifunctional Oxygen Electrocatalysts in Alkaline Media. ACS Appl. Mater. Interfaces 2018, 10, 20440–20447.
- Zhang, K.; Zhang, L.; Chen, X.; He, X.; Wang, X.; Dong, S.; Gu, L.; Liu, Z.; Huang, C.; Cui, G. Molybdenum Nitride/N-Doped Carbon Nanospheres for Lithium-O2 Battery Cathode Electrocatalyst. ACS Appl. Mater. Interfaces 2013, 5, 3677–3682.
- Chen, Y.; Li, Z.; Zhu, Y.; Sun, D.; Liu, X.; Xu, L.; Tang, Y. Atomic Fe Dispersed on N-Doped Carbon Hollow Nanospheres for High-Efficiency Electrocatalytic Oxygen Reduction. Adv. Mater. 2019, 31, e1806312.
- Feng, Q.; Xiong, Y.; Xie, L.; Zhang, Z.; Lu, X.; Wang, Y.; Yuan, X.-Z.; Fan, J.; Li, H.; Wang, H. Tungsten Carbide Encapsulated in Grape-Like N-Doped Carbon Nanospheres: One-Step Facile Synthesis for Low-Cost and Highly Active Electrocatalysts in Proton Exchange Membrane Water Electrolyzers. ACS Appl. Mater. Interfaces 2019, 11, 25123–25132.
- Zhu, J.; Abdelkader, A.; Demko, D.; Deng, L.; Zhang, P.; He, T.; Wang, Y.; Huang, L. Electrocatalytic Assisted Performance Enhancement for the Na-S Battery in Nitrogen-Doped Carbon Nanospheres Loaded with Fe. Molecules 2020, 25, 1585.
- Chi, J.-Q.; Gao, W.-K.; Zhang, L.-M.; Dong, B.; Yan, K.-L.; Lin, J.-H.; Liu, B.; Chai, Y.-M.; Liu, C.-G. Induced Phosphorization-Derived Well-Dispersed Molybdenum Phosphide Nanoparticles Encapsulated in Hollow N-Doped Carbon Nanospheres for Efficient Hydrogen Evolution. ACS Sustain. Chem. Eng. 2018, 6, 7676–7686.
- Gavrilov, N.; Pašti, I.A.; Vujković, M.; Travas-Sejdic, J.; Ćirić-Marjanović, G.; Mentus, S.V. High-performance charge storage by N-containing nanostructured carbon derived from polyaniline. Carbon 2012, 50, 3915–3927.
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191.
- Hass, J.; de Heer, W.A.; Conrad, E.H. The growth and morphology of epitaxial multilayer graphene. J. Phys. Cond. Matt. 2008, 20, 323202.
- Wang, C.; Zhou, Y.; He, L.; Ng, T.; Hong, G.; Wu, Q.-H.; Gao, F.; Lee, C.-S.; Zhang, W. In situ nitrogen-doped graphene grown from polydimethylsiloxane by plasma enhanced chemical vapor deposition. Nanoscale 2013, 5, 600–605.
- Gao, Y.; Hu, G.; Zhong, J.; Shi, Z.; Zhu, Y.; Su, D.S.; Wang, J.; Bao, X.; Ma, D. Nitrogen-Doped sp2-Hybridized Carbon as a Superior Catalyst for Selective Oxidation. Angew. Chem. Int. Ed. 2013, 52, 2109–2113.
- Jeong, H.M.; Lee, S.Y.; Shin, W.H.; Kwon, J.H.; Shakoor, A.; Hwang, T.H.; Kim, S.Y.; Kong, B.-S.; Seo, J.-S.; Lee, Y.M.; et al. nitrogen-doped carbon spheres through a bottom-up approach are highly robust lithium-ion battery anodes. RSC Adv. 2012, 2, 4311–4317.
- Li, Y.; Zhao, Y.; Cheng, H.; Hu, Y.; Shi, G.; Dai, L.; Qu, L. Nitrogen-Doped Graphene Quantum Dots with Oxygen-Rich Functional Groups. J. Am. Chem. Soc. 2012, 134, 15–18.
- Jeong, H.M.; Lee, J.W.; Shin, W.H.; Choi, Y.J.; Shin, H.J.; Kang, J.K.; Choi, J.W. Nitrogen-Doped Graphene for High-Performance Ultracapacitors and the Importance of Nitrogen-Doped Sites at Basal Planes. Nano Lett. 2011, 11, 2472–2477.
- Jin, Z.; Yao, J.; Kittrell, C.; Tour, J.M. Large-Scale Growth and Characterizations of Nitrogen-Doped Monolayer Graphene Sheets. ACS Nano 2011, 5, 4112–4117.
- Wei, D.; Liu, Y.; Wang, Y.; Zhang, H.; Huang, L.; Yu, G. Synthesis of N-Doped Graphene by Chemical Vapor Deposition and Its Electrical Properties. Nano Lett. 2009, 9, 1752–1758.
- Wang, Y.; Shao, Y.; Matson, D.W.; Li, J.; Lin, Y. Nitrogen-Doped Graphene and Its Application in Electrochemical Biosensing. ACS Nano 2010, 4, 1790–1798.
- Zhang, C.; Fu, L.; Liu, N.; Liu, M.; Wang, Y.; Liu, Z. Synthesis of Nitrogen-Doped Graphene Using Embedded Carbon and Nitrogen Sources. Adv. Mater. 2011, 23, 1020–1024.
- Li, X.; Wang, H.; Robinson, J.T.; Sanchez, H.; Diankov, G.; Dai, H. Simultaneous Nitrogen Doping and Reduction of Graphene Oxide. J. Am. Chem. Soc. 2009, 131, 15939–15944.
- Sheng, Z.-H.; Shao, L.; Chen, J.-J.; Bao, W.-J.; Wang, F.-B.; Xia, X.-H. Catalyst-Free Synthesis of Nitrogen-Doped Graphene via Thermal Annealing Graphite Oxide with Melamine and Its Excellent Electrocatalysis. ACS Nano 2011, 5, 4350–4358.
- Yang, S.-Y.; Chang, K.-H.; Huang, Y.-L.; Lee, Y.-F.; Tien, H.-W.; Li, S.-M.; Liu, C.-H.; Ma, C.-C.M.; Hu, C.-C. A powerful approach to fabricate nitrogen-doped graphene sheets with high specific surface area. Electrochem. Commun. 2012, 14, 39–42.
- Mayavan, S.; Sim, J.-B.; Choi, S.-M. Easy synthesis of nitrogen-doped graphene–silver nanoparticle hybrids by thermal treatment of graphite oxide with glycine and silver nitrate. Carbon 2012, 50, 5148–5155.
- She, Y.; Chen, J.; Zhang, C.; Lu, Z.; Ni, M.; Sit, P.H.-L.; Leung, M.K.H. Nitrogen-doped graphene derived from ionic liquid as metal-free catalyst for oxygen reduction reaction and its mechanisms. Appl. Energy 2018, 225, 513–521.
- Panchakarla, L.S.; Subrahmanyam, K.S.; Saha, S.K.; Govindaraj, A.; Krishnamurthy, H.R.; Waghmare, U.V.; Rao, C.N.R. Synthesis, Structure, and Properties of Boron- and Nitrogen-Doped Graphene. Adv. Mater. 2009, 21, 4726–4730.
- Hulicova, D.; Yamashita, J.; Soneda, Y.; Hatori, A.H.; Kodama, M. Supercapacitors Prepared from Melamine-Based Carbon. Chem. Mater. 2005, 17, 1241–1247.
- Yang, H.B.; Miao, J.; Hung, S.-F.; Chen, J.; Tao, H.B.; Wang, X.; Zhang, L.; Chen, R.; Gao, J.; Chen, H.M.; et al. Identification of catalytic sites for oxygen reduction and oxygen evolution in N-doped graphene materials: Development of highly efficient metal-free bifunctional electrocatalyst. Sci. Adv. 2016, 2, e1501122.
- Liu, Y.; Zhao, J.; Cai, Q. Pyrrolic-nitrogen doped graphene: A metal-free electrocatalyst with high efficiency and selectivity for the reduction of carbon dioxide to formic acid: A computational study. Phys. Chem. Chem. Phys. 2016, 18, 5491–5498.
- Ju, M.J.; Kim, J.C.; Choi, H.-J.; Choi, I.T.; Kim, S.G.; Lim, K.; Ko, J.; Lee, J.-J.; Jeon, I.-Y.; Baek, J.-B.; et al. N-Doped Graphene Nanoplatelets as Superior Metal-Free Counter Electrodes for Organic Dye-Sensitized Solar Cells. ACS Nano 2013, 7, 5243–5250.
- Rahsepar, M.; Nobakht, M.R.; Kim, H.; Pakshir, M. Facile enhancement of the active catalytic sites of N-doped graphene as a high performance metal-free electrocatalyst for oxygen reduction reaction. Appl. Surf. Sci. 2018, 447, 182–190.
- Maouche, C.; Zhou, Y.; Li, B.; Cheng, C.; Wu, Y.; Li, J.; Gao, S.; Yang, J. Thermal treated three-dimensional N-doped graphene as efficient metal free-catalyst for oxygen reduction reaction. J. Electroanal. Chem. 2019, 853, 113536.
- Kumar, M.P.; Raju, M.M.; Arunchander, A.; Selvaraj, S.; Kalita, G.; Narayanan, T.N.; Sahu, A.K.; Pattanayak, D.K. Nitrogen Doped Graphene as Metal Free Electrocatalyst for Efficient Oxygen Reduction Reaction in Alkaline Media and Its Application in Anion Exchange Membrane Fuel Cells. J. Electrochem. Soc. 2016, 163, F848.
More
