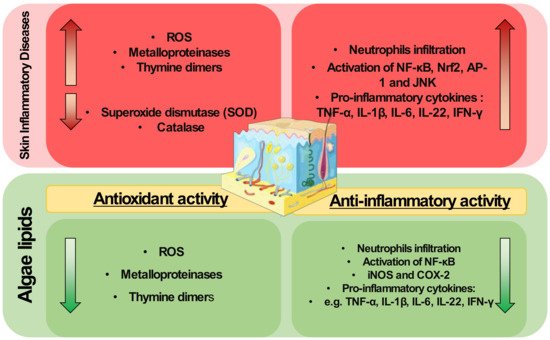
Lipids from algae have been scarcely applied to modulate skin diseases, but they are well known antioxidant and anti-inflammatory agents. They have shown scavenging activities and can modulate redox homeostasis enzymes. They can also downmodulate key inflammatory signaling pathways and transcription factors such as NF-κB, decreasing the expression of pro-inflammatory mediators. Thus, the exploitation of algae lipids as therapeutical agents for the treatment of inflammatory skin diseases is highly attractive.
- skin diseases
- inflammation
- oxidative stress
- lipidomics
- bioactive lipids
- anti-inflammatory
- antioxidant
- macroalgae
- microalgae
1. Introduction
2. Algae Lipids with Antioxidant Activity
The antioxidant activity of algae occurring lipids has been studied, but is much less explored compared to well-known natural antioxidants such as pigments and phenolic compounds [17][18][67,68] (Table 1).| Studies | Mechanism |
|---|
| Studies | Assay | Identified Lipids | Algae Species | Ref. | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Action | Model | Identified Lipids | Algae Species | Ref. | ||||||||||||
| in chemico | Free radical scavenging | ABTS, DPPH, hydroxyl radical, superoxide anion | Polar lipids, neutral lipids, FAME | |||||||||||||
| In chemico | COX-2 inhibition | COX-2 kit assay | Macroalgae: | Bifurcaria bifurcata, Codium tomentosum, Fucus vesiculosus, Gracilaria gracilis Grateloupia turuturu, Palmaria palmata, Porphyra dioica Sargassum muticum, Solieria chordalis, Ulva rigida | Microalgae: | Chlorella vulgaris, Chlorococcum amblystomatis, Nannochloropsis oceanica, Phaeodactylum tricornutum, Scenedesmus intermedius Scenedesmus obliquus, Spirulina | sp., | Tetraselmis chui | Polar lipids | Macroalgae: | Codium tomentosum, Fucus vesiculosus Gracilaria gracilis, Palmaria palmata, Porphyra dioica, Ulva rigida, | Microalgae: | Chlorella vulgaris, Chlorococcum amblystomatis, Gloeothece sp., Skeletonema | sp., | [19][20][21][22] | [69,70,82,83] |
| Tetraselmis sp. mutants | [ | 19 | ] | [ | 32][33][34][35] | [69,92,93,94,95] | in vitro | Detoxify intracellular ROS |
Increased the expression of Nrf2 in irradiated HaCat cells Upregulate target antioxidant enzymes Cu/Zn SOD, CAT, and HO-1 |
Crude ethanolic extract | ||||||
| In vitro | NO inhibition | Macroalga: | Raw 264.7 | Carpomitra costata | Polar and non-polar lipids; PC, PG, DGDG, DGTS, MGDG, MGMG, SQDG classes; Free and ethyl esterified DGLA | [23 | Macroalgae: | Chondrus crispus, Lobophora sp.Palmaria palmata | , Microalgae: | Chlorella sorokiniana Lobosphaera incisa, Nannochloropsis granulata, Tetraselmis chui,] | [84] | |||||
| [ | 36 | ] | [ | 37 | ][38][39][40][41][42][43] | [96,97,98,99,100,101,102,103] | Free radical scavenging | Superoxide generation on peritoneal leukocytes | ||||||||
| Decrease in PGE2 Downregulation of COX-2 | Raw 264.7; White blood cells; Epidermal cells | Sulfoquinovosylacylglycerols | Crude ethanolic extracts; Microalgae: | Porphyridium cruentum | lipid extracts rich in PC; free and ethyl esterified DGLA | [ | Macroalgae: | Laminaria ochroleuca | Microalgae: | Chlorella vulgaris, Chloromonas reticulata, Lobosphaera incisa Micractinium | sp., | Phaeodactylum tricornutum,24] | [86] | |||
| [ | 41 | ] | [ | 44][45][46][47][48] | [101,104,105,106,107,119] | Inhibition of ROS | Photoprotective against UVB in NHDF | Crude ethyl acetate extract | Microalga: | Ettlia | sp. YC001 | [ | ||||
| Downregulation of mRNA expression of pro-inflammatory cytokines Downregulation of cytokines levels: TNF-α, IL-6, IL-1α, and IL-1β |
THP-1; PBMC; Epidermal cells; HaCaT cells |
Crude ethanolic extracts; lipid extracts; lipid extracts rich in MGDG, DGDG and SQDG; Lipid extracts rich in PC; LPC(16:0); oxylipins; ergosterol and 7-dehydroporiferasterol; free and ethyl esterified DGLA |
Macroalgae: | Chondrus crispus, Laminaria ochroleuca, Palmaria palmata, Porphyra dioica, Prasiola japonica | Microalgae: | Aurantiochytrium mangrovei, Chlamydomonas debaryana, Chlorella vulgaris, Chloromonas reticulata, Cylindrotheca closterium, Dunaliella tertiolecta, Micratinium | sp., | Nannochloropsis gaditana, Nitzschia palea, Phaeodactylum tricornutum, Lobosphaera incisa Spirulina maxima, Pavlova lutheri, Tetraselmis suecica,25] | [85] | |||||||
| [ | 23 | ] | [ | 41][44][45][46][47][49][50][51][52][53][57][58] | Enzyme/protein expression | Downregulation of expression of MMPs | Crude ethanolic extract | Microalga: | Arthrospira platensis | [26] | [87] | |||||
| Enzyme/protein expression | Downregulation of expression of MMPs, IL-6 and TGF-1 in human dermal fibroblast Modulate MAPK in irradiated HaCat cells |
Fucosterol | Macroalga: | Sargassum fusiforme | [27][28] | [88,89] |
The pathophysiology of inflammatory skin diseases is associated with unregulated elevated levels of ROS and the activity of enzymes and proteins involved in the regulation of oxidative stress [29][30]. In cells, mitochondria metabolize oxygen, producing ROS. During the oxidative phosphorylation in mitochondria, oxygen is converted to O2•−, which can be transformed in H2O2 by superoxide dismutase, and then to water by glutathione peroxidase (GPX) or peroxiredoxin III (PRX III) radical [30][90]. Under normal conditions, the mitochondria ROS production is balanced by the production of a variety of antioxidants. However, oxidative stress occurs when there is an imbalance between ROS and antioxidants production. An imbalance in ROS production leads to redox signaling from cellular organelles, causing mitochondrial damage and dysfunction in several conditions [31][91]. However, the application of algae lipids to prevent mitochondrial dysfunction and modulate the oxidative status is little understood and requires in-depth study to understand the mechanisms underlying this potential antioxidant role. The use of crude extracts from algae may reduce ROS levels induced by UVB and impair the expression of MMPs and thymine dimers formation due to UVB exposure in skin cells [26][87]. These studies were performed using complex crude extracts rich in lipids and not with isolated lipids or fractions. This hinders the understanding of the mechanisms of action of algal lipids as antioxidants, and more work is needed to determine the potential protective role of algal lipids in skin diseases. A better understanding of this antioxidant action is needed, for example, there is a lack of knowledge about the impact of specific lipid classes or lipid molecules in the enzymes and proteins involved in the regulation of oxidative stress, such as metalloproteinases, HO-1, catalase, or superoxide dismutase.
3. Algae Lipids with Anti-Inflammatory Activity
The lipids of macro- and microalgae have been studied for their anti-inflammatory and immunomodulatory activity. Most of the studies tested crude lipid extracts or fractions of lipid classes of algae and were mainly performed in chemico and in vitro (Table 2). They mainly measured the impact of lipids on the levels of inflammatory effector molecules such as prostaglandins and nitric oxide (NO), cytokines such as TNF-α, IL-6, and IL-1β, and on the activation of inflammatory signaling pathways (NF-κB) or cyclooxygenase-2 (COX-2) activity. Few studies have evaluated in vivo models measuring the effect of lipids on skin cells, such as epidermal cells.| [ | ||||||||||||
| 84 | ||||||||||||
| , | ||||||||||||
| 101 | ||||||||||||
| , | 104 | [ | 54 | ,105 | ] | ,106 | [ | ,107,111,112,113,114,115 | 55][56][48 | ,110 | ] | ,116,118,119,121,122] |
| Inhibition of pro-inflammatory signaling pathways mediated by TLR and NF-κB | THP-1 | Lipid extracts rich in MGDG, DGDG, and SQDG | Macroalgae: | Chondrus crispus, Palmaria palmata, Porphyra dioica | Microalgae: | Pavlova lutheri | [49] | [110] | ||||
| In vivo | Attenuation of ear oedema | PLA2 kit assay; Mice with ear oedema; DNFB-induced in naive C57BL/6 mice |
MMHDA; Lipid extracts rich in PC; MGDG, DGDG, and SQDG fractions |
Macroalgae: | Ishige okamurae | , | Laminaria ochroleuca | Microalgae: ETS-05 cyanobacterium. |
[48][59][60] | [119,123,127] | ||
| Neutrophil gathering in the wound region | Wounded zebrafish model | Glycolipids rich in γ-linolenic acid | Microlagae: | Spirulina platensis | [61] | [124] | ||||||
| Inhibition of pro-inflammatory cytokines production: TNF-α, IL-6, IL-8, IFN- γ, IL-1β, IL-17 | db/db and CD1 mice model of diabetes mellitus; TNBS-induced colitis rats; BALB/c mice skin; TPA-induced hyperplasia murine model |
Crude ethanolic extract; omega-3 fatty acids; oxylipins; MGDG cream |
Macroalgae: | Sargassum cristaefolium | Microalgae: | Chlamydomonas debaryana, Isochrysis galbana | [62][63][64][65] | [125,126,128,129] | ||||
| Downregulation of iNOS and COX-2, and decrease in NO and PGE2 production | TNBS-induced colitis rat; BALB/c mice skin; TPA-induced hyperplasia murine model |
Crude ethanolic extract; oxylipins; MGDG cream | Macroalgae: | Sargassum cristaefolium | Microalgae: | Chlamydomonas debaryana, Isochrysis galbana | [63][64][65] | [126,128,129] |
It was demonstrated that methoxylated fatty acids (MMHDA) isolated from macroalga Ishige okamurae [60][127] and MGDG, DGDG, and SQDG fractions from microalga ETS-05 cyanobacterium [59][123] presented anti-inflammatory activity by reducing ear oedema (swelling) in a mouse model. The anti-inflammatory action of MMHDA has been associated with the inhibition of phospholipase A2 (PLA2), the enzyme responsible for the hydrolysis of the sn-2 position of membrane glycerophospholipids to liberate arachidonic acid (AA). The reduction in neutrophils was observed in the wound region of a zebrafish model when glycolipids rich in γ-linolenic acid from the microalga Spirulina platensis were used [61][124]. Extracts with omega-3 FA isolated from microalgae promoted the reduction of CD4+ T cells production of the pro-inflammatory mediators IFN-γ, TNF-α, and IL-4 and increased the secretion of IL-17A, IL-14, and TGF-β in a db/db and CD1 mouse model of diabetes Mellitus [62][125]. Downregulation of TNF-α was also observed, as well as decreased expression of iNOS and COX-2, when 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced colitis rats were supplemented with oxylipins extracted from Chlamydomonas debaryana [63][126].
In model studies on skin diseases, the protective effect of the ethanolic extract of Sargassum cristaefolium against ultraviolet-irradiated skin keratinocytes and BALB/c mice skin has been demonstrated [64][128]. The inhibition of ROS production and suppression of the apoptotic process in irradiated cells, such as the decrease of caspases, downregulation of COX-2, IL-1β, IL-8, IL-6, TNF-α, and INF-γ, and down-modulation of NF-κB signaling, were some of the reported mechanisms of action. L. ochroleuca lipid extract have been shown to reduce ear oedema, in a murine model of skin inflammation induced by the chemical sensitizer 2,4-dinitro-fluorobenzene applied to the ear of naive C57BL/6 mice [48][119]. A cream containing MGDG extracted from the microalga Isochrysis galbana has been reported to have beneficial effects in the treatment of a 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced hyperplasia murine model [65][129]. Pre-treatment of these mice with this MGDG cream reduced skin oedema and epidermal thickness. In addition, the pro-inflammatory cytokines TNF-α, IL-1β, IL-6, and IL-17 produced in epidermal tissue were downregulated and the expression of COX-2 was inhibited. The results of this study were very promising as they involved a model of skin disease and isolated algal lipids and showed a strong anti-inflammatory effect as well as an improved skin condition. Such results highlight algal lipids as promising pharmacological strategies for the therapy of inflammatory skin pathologies. Algal lipids have shown anti-inflammatory potential as modulators of signaling pathways and mediators, known as the main hallmarks of inflammation, such as COX-2 and iNOS, but also the modulation of the production of cytokines (TNF-α, IL-6, IL-1β, and IFN-γ), as described in Figure 1. Indeed, as mentioned in Section 2, inflammatory skin diseases are characterized by systemic inflammation [66][130] with the infiltration of immune cells, such as neutrophils. Algal lipids such as glycolipids rich in γ-linolenic acid from the microalga S. platensis have been reported to reduce neutrophil infiltration in a wound region model of zebrafish [61][124]. Neutrophils are the main producers of ROS capable of activating transcription factors, such as NF-κB, responsible for the regulation of genes involved in inflammation [67][43]. Interestingly, the use of extracts in immune and skin cell lines reduced this pro-inflammatory pathway [49][50][59][110,111,123]