The problems of the annual formation of industrial waste are common to a wide group of industries, particularly chemical, petrochemical, coal, gas, and wood processing. The most typical wastes of these industries are coal tar, waste oils, oil sludge, filter cakes, coal slime, sawdust, wood shavings, etc. Most of these materials and components pose a significant environmental threat. A successful solution to these problems is possible due to the use of auxiliary fuel; boiler modifications; oxy-fuel combustion; and the preparation of multi-component fuels, including the use of additives.
1. Introduction
The problems of the annual formation of industrial waste are common to a wide group of industries, particularly chemical, petrochemical, coal, gas, and wood processing
[1][2][3][1,2,3]. The most typical wastes of these industries are coal tar, waste oils, oil sludge, filter cakes, coal slime, sawdust, wood shavings, etc.
[4][5][6][4,5,6]. Most of these materials and components pose a significant environmental threat. Waste occupies large areas and penetrates soil and water; gradual thermochemical transformation of waste is accompanied by the release of hazardous substances
[7][8][7,8]. The most common methods of industrial waste disposal are the following
[9][10][11][9,10,11]: burial, removal of impurities, storage, and reuse for its intended purpose, use as secondary raw material in oil refining and coal preparation, pelletizing, pyrolysis, gasification, and combustion. Most of the treatment and cleaning methods are quite ineffective for large volumes of industrial waste
[5][9][10][5,9,10]. At the same time, many enterprises are forced to incur heavy losses due to environmental fines
[12] associated with ineffective waste disposal or its absence. Disruptive technologies are required for efficient waste disposal. However, their creation and adaptation require significant economic costs at the initial stage.
Municipal waste is no less dangerous for humanity. In terms of component composition, accumulated volumes, and rates of annual formation, they are practically not inferior to industrial ones
[13]. In countries with undeveloped economies, municipal solid waste is considered even more hazardous than industrial waste. The most typical municipal solid waste includes cardboard, paper, plastic, polyethylene, rubber, food debris, etc.
[14][15][14,15]. Landfilling, thermal treatment, and incineration with energy generation are popular disposal methods for such waste
[16][17][18][16,17,18]. Holubčík et al.
[19] used slow pyrolysis of shredded used car tires and plastic packaging. The research
[19] has confirmed that pyrolysis allows for the production of valuable products with minimal damage to the environment. Bala-Litwiniak and Radomiak
[20] have shown that waste glycerol can be successfully used as a fuel in combination with wood pellets. Glycerin with a fraction of no more than 4.5% improved the quality of the pellets and the environmental performance
[20]. Dudyński et al.
[21] carried out a test gasification of leather waste on a laboratory and industrial scale. As a result, a producer gas was obtained with a heating value of 4.1–6.5 MJ/m
3. Dudyński et al.
[21] concluded that gasification of waste leather may be more promising than incineration, mainly due to greater environmental safety.
However, the rate of the annual increase in municipal waste is so high that the factories for their utilization manage to process no more than 20–30%. The main difficulty lies in the need to sort waste to ensure high economic performance. Unfortunately, in many regions, management mechanisms and regulatory documents have not been formed for the effective separation of waste. As a result, numerous landfills increase in volume every year. Waste disposal technologies without preliminary sorting are important.
Analysis of the current state of utilization of industrial and municipal waste
[16][22][16,22] shows that technologies are required that allow for solving a set of problems. In particular, it is necessary, along with waste disposal, to effectively expand the raw material base, reduce the anthropogenic load on the environment, and increase the area for beneficial use.
We need objective assessments of the totality of economic, environmental, energy, and social criteria and justification of the effectiveness of technologies, taking into account all the main categories (for example, using methods of multiple-criteria decision analysis [23,24]). The development of universal technological solutions for preparing waste for incineration or deep conversion, storage, transportation under different climatic conditions, and spraying in combustion chambers is of current interest. To solve this kind of problem, it is important to analyze modern ideas about the relevant processes, including the results of experimental and theoretical studies of the world scientific community. To date, a large experimental base has been obtained
[23][24][25,26] and the results of mathematical modeling
[25][26][27,28], which develop ideas from reviews
[27][28][29][29,30,31] and books
[30][31][32,33].
2. Main Types of Combustible Waste
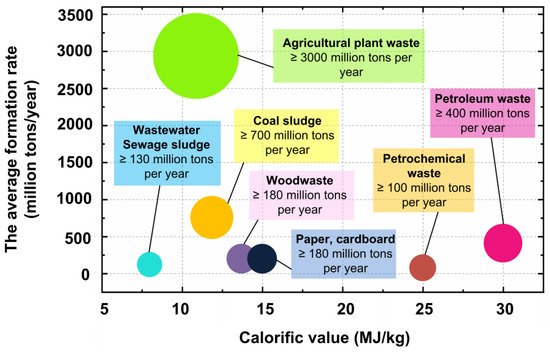
The annual world production of waste is at least 4500 million tons. Among the main sources of waste are the following: (i) energy sector (waste from the production, processing, and combustion of fuels); (ii) industry (waste oils and chemicals, machines and mechanisms) municipal sector (solid waste, sewage sludge, construction waste); woodworking and agricultural sector (sawmill waste, woodworking, agricultural waste, etc.). Each of the listed categories of waste contains wastes that are suitable for the preparation of mixed and slurry fuels (
Figure 1).
Figure 1. Calorific value of wastes and their average global generation rate (according to [13][32][33][34][35]). Calorific value of wastes and their average global generation rate (according to [13,34,35,36,37]).
Coal of different grade
[36][37][38,39] and coal slimes
[38][39][40,41] can be used as basic components. The water of different quality (polluted or purified)
[40][42], wastewater
[41][43], and industrial water
[36][38] can be used as a dispersed medium. Additional components (usually in a small amount of 5–20 wt%) can be the following: oil sludge
[42][43][44,45], used automotive and industrial oils
[37][44][39,46], alcohols
[45][46][47,48], and biomass
[44][47][46,49]. Such additives are used to improve the ignition and combustion performance of the fuel, increase its calorific value, and reduce emissions. Solid components can be torrefied to improve efficiency. This method is a promising thermochemical technology for converting solid feedstock (most often biomass) into biochar for co-combustion
[48][49][50,51] or for pyrolysis or gasification
[50][51][52,53]. Torrefaction is defined as thermal treatment in an inert environment at atmospheric pressure and temperatures within the range of 200–300 °C. The main principles of torrefaction are to remove oxygen, reduce moisture content, and produce a solid residue that has a lower O/C ratio than the feedstock. The main purpose of torrefaction is to increase the calorific value. The main product of torrefaction is solid biochar (up to 80 wt% of feedstock)
[50][52].
Table 1 provides a list of typical components used to create fuel slurries and blends.
Table 1. Properties of the components used for the preparation of fuel mixtures.
|
Component
|
Ultimate Analysis (wt%)
|
Proximate Analysis (wt%)
|
Ref.
|
|
C
|
H
|
O
|
N
|
S
|
Moisture
|
Volatile Matter
|
Fixed Carbon
|
Ash
|
Calorific Value
(MJ/kg)
|
|
Shenhua coal
|
69.55
|
3.74
|
10.14
|
0.83
|
0.25
|
8.28
|
29.55
|
54.96
|
7.21
|
27.07
|
[36] |
|
|
[ |
61 |
] |
[ |
63 |
] |
|
|
|
|
Waste lubricating oil |
|
|
|
| 84.02
|
13.31
|
1.92
|
-
|
0.75
|
-
|
-
|
-
|
-
|
-
|
[44][46]
|
|
Waste cooking oil
|
71.84
|
10.14
|
17.71
|
0.06
|
0.01
|
0.08
|
99.15
|
0.56
|
0.24
|
39.24
|
[62][64]
|
|
Oily sludge
|
63.9
|
7.3
|
25.3
|
1.2
|
2.3
|
33.4
|
69.3
|
-
|
21.2
|
23
|
[42][44]
|
|
Bio-oil (from pyrolysis of pine)
|
41.47
|
6.37
|
52.05
|
0.11
|
-
|
24.7
|
73.1
|
2.1
|
0.1
|
16.9
|
[63][65]
|
|
Corn stalk
|
32.01
|
3.44
|
24.0
|
1.02
|
0.22
|
6.77
|
52.1
|
8.61
|
32.52
|
11.87
|
[64][66]
|
|
Coal slime
|
53.29
|
3.89
|
9.41
|
0.83
|
0.65
|
0.95
|
27.51
|
36.62
|
34.92
|
22.07
|
[57][59]
|
|
Bamboo residual
|
55.51
|
6.12
|
42.05
|
0.21
|
0.11
|
-
|
-
|
-
|
-
|
-
|
[44][46]
|
|
Corn silage
|
43.40
|
6.17
|
46.70
|
1.02
|
0.93
|
-
|
-
|
-
|
-
|
-
|
[65][67]
|
|
Clover grass
|
44.90
|
6.8
|
43.30
|
2.2
|
0.3
|
-
|
-
|
-
|
-
|
-
|
[65][67]
|
|
Biochar (from pyrolysis of pine)
|
86.83
|
3.34
|
9.7
|
0.13
|
-
|
2.4
|
16.4
|
80.6
|
3.0
|
28.3
|
[63][65]
|
3. Combustion of Non-Conventional Liquid, High-Moisture, and Slurry Fuels
Most studies on the incineration of waste and low calorific fuels involve the use of solid, specially treated, and dehydrated components (for example,
[6][47][66][6,49,68]). Co-firing of coal and biomass
[67][69], as well as the individual firing of biomass, are most actively studied. This is partly because the energy use of biomass is already reaching an industrial level in many countries and requires large-scale tests
[67][68][69,70]. To study the individual and co-combustion of biomass and solid waste, quite a few types of plants are used, including reactors and furnaces of both laboratory
[69][71] and pilot scale
[70][72]. The number of papers on the regularities of combustion of liquid fuels (oils, slurries, and emulsions based on waste) is much less.
When studying the thermal properties of mixed fuels and individual components, standard methods are widely used (thermogravimetric analysis, calorimetry, spectrometry, etc.). The characteristics of ignition and burnout of fuels, depending on the research objectives, are studied using installations of various types and power (some typical examples are given in
Table 2).
Table 2. Experimental plants for the study of ignition and combustion of non-conventional fuels.
Studies of pyrolysis and gasification of mixed fuels carried out via pilot, laboratory, and industrial installations.
|
Fuel
|
Process
|
Temperature Conditions
|
Ref.
|
Characteristics of the Plant |
|
| Temperature
|
Key Result
|
Ref.
|
|---|
|
Stem wood, bark, forest residue, willow, and reed canary grass and pyrolysis oil and solid residue from them
|
Tube furnace blown by gas mixtures (air, N |
|
Coal–oil–water slurry (COWS)
(coal 45–55 wt%, oil 10–20 wt%; water 35 wt%)
| 2, O2)
|
|
Pyrolysis
<1400 °C
|
Laboratory tube furnace.
The carrier gas: N2, flow rate 0.8 L/min.
[71][73]
|
Experiment time: 30 min. Particle size: 75–100 μm. |
|
| 800, 900 and 1000 °C
|
An increase in the temperature and the proportion of water in the fuel contributed to an increase in the gas yield up to 2.8 times, while the char yield decreased to 1.4 times. The addition of waste oil resulted in a decrease in CO and CO2, and an increase in CH4 and H2.
Pyrolysis gas composition: H2: 80–270 mL/g; CO: 35–110 mL/g; CO2: 22–120 mL/g; CH4: 60–150 mL/g.
|
[60][62]
| [ | 38 | ] |
|
|
Samca coal
|
Emulsion based on water and heating oil; slurry based on water and pyrolytic soot
75.9
|
5.3
|
12.27
|
|
|
Coal wastewater slurry (CWWS)
(coal 57.2–62 wt%, water 42.8–38 wt%). |
| 0.7 |
|
Chamber with industrial burners with a total power of 1.2 MW
|
Gasification
Temperature of flue gases > 1100 °C
Maximum operating temperature 1430 °C
|
[53][55]
|
|
| Industrial CWS gasifier to produce syngas and synthesize ammonia. |
Syngas output 515,116.8 m 3/day.
Particle size: 40 μm.
|
|
1350–1400 °C
| 5.8
|
The syngas produced by the CWWS gasification has a higher effective gas component (CO + H2) than the CWS. In addition, the use of a waste-based slurry increased cold gas efficiency by 1.57% and carbon conversion by 0.45% in industrial processes.
Syngas composition: H2: 30.5%; CO: 48.1%; CO2: 16.3%; CH4: 0.9%; N2
-
|
: 4.2%.
|
[36][38]
36.9
|
-
|
22.8
|
-
|
[37][39]
|
|
Coal gangue
|
Spherical particles of corn stalk and bituminous coal
17.5
|
|
|
Waste oil/coal slurry (coal 50 wt%, mineral waste oil 50 wt%).
|
Reactor (electrical quartz tube), blown by mixtures of O2/N2 and O2/H2O
|
1.26
|
Pyrolysis
800 °C
-
|
[64][66]
|
|
| Laboratory fluidized bed reactor. |
Feeding rate 550 g/h. Fuel mass 3 kg.
|
625 °C
|
0.56
|
The quality of waste oil/coal slurry pyrolysis products was higher compared to coal pyrolysis products. During the slurry pyrolysis, the gas yield increased from 14.2% to 31.6%, and the liquid yield increased from 17.4 to 29.1% in comparison with coal. At the same time, the concentrations of CH4, H2, C2H4, and C2H6 increased by 3.3, 2.5, 32, and 10 times, respectively.
Pyrolysis gas composition:
H2: 0.5 wt%; CO: 1.6 wt%; CO2
1.28
|
: 3.4 wt%; CH
0.75
|
4
15.07
|
16.31
|
68.62
|
4.82
|
[38][40]
|
|
Coal slime
|
Sewage sludge with coal–water slurry (CWS)
87.2
|
Large scale fluidized bed incinerator
5.1
|
>1000 °C
4.5
|
|
[56]
2.1
|
[
1.1
|
58
-
|
]
23.1
|
-
|
26.5
|
24.83
|
[39][41]
|
|
Semicoke powders
|
|
|
Wet sewage sludge with wood chips
| 69.12
|
Grate-fired boiler with a vibrating grate
1.35
|
|
>1000 °C
10.33
|
[72][74]
0.89
|
|
|
Pyrolysis oil from sewage sludge, heavy fuel oil
|
Laboratory setup with heat sources in the form of two plates
|
Temperature of the plates is 500, 550, 600 °C
|
[58][60]
|
] | [ | 48 | ] |
|
Slurry based on coal, water and waste soot
|
Rotary kiln
|
800 °C
|
[55][57]
|
|
Slurries based on coal and liquid waste from petrochemical industry
|
Pilot-scale combustion system
|
1100–1300 °C at steady combustion
|
[73][75]
|
4. Waste Conversion for Fuel Gas Production
Gasification and pyrolysis are environmentally promising waste treatment technologies, as they produce less pollution in comparison with combustion, in particular, by SO
x and NO
x emission
[74][90]. Currently, a significant number of studies have been carried out on pyrolysis and gasification of conventional energy sources such as coal
[75][76][77][91,92,93] and biomass
[78][79][80][94,95,96]. However, the methods of thermal conversion of mixed waste-derived fuels to obtain fuel gas and other valuable pyrolysis products (char, oil) are less studied.
The following sections of the article provide an overview of studies on pyrolysis and gasification of mixed and slurry fuels prepared based on wastes of different origins.
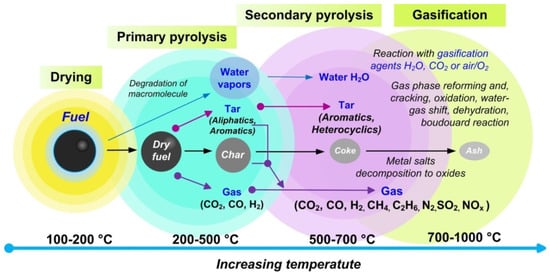
Figure 24 shows typical stages that occur during pyrolysis and gasification of fuels
[74][81][90,97]. When a fuel particle is introduced into a heated medium, heating of the particle is observed, which intensifies moisture evaporation (drying stage). First, the external, unbound moisture evaporates, and then the internal one begins to evaporate in a quasi-stationary mode. After reaching the critical moisture content, the drying rate begins to decrease. After drying, the stage of primary pyrolysis follows, which is characterized by the release of volatile pyrolytic substances. Primary volatiles are formed as a result of the thermal rupture of the chemical bonds of individual fuel constituents. These include permanent gas particles (e.g., CO
2, CO, H
2), ambient organic compounds (aliphatic and aromatic), and water. In addition to the listed substances, at this stage, a non-volatile carbon-riched solid residue (char) is formed. The resulting char contains a significant proportion of the minerals of the original fuel. In general, the primary pyrolysis stage is completed at temperatures of about 500 °C. With a subsequent temperature increase, a part of the primary volatiles is involved in a variety of reactions of secondary pyrolysis (500–700 °C) and gasification (700–1000 °C).
Figure 24.
Scheme of thermal decomposition of a fuel particle indicating the main stages of pyrolysis and gasification.
However, there are no clear borders between primary and secondary pyrolysis
[81][97] since secondary reactions of volatiles can occur simultaneously both in the pores of particles and in the volume of the gas. At high temperatures, sequential and parallel reactions proceed (heterogeneously or homogeneously), for example, cracking, reforming, dehydration, condensation, polymerization, oxidation, and gasification reactions. Under these conditions, char can be converted into gaseous particles during gasification reactions in an H
2O atmosphere (which is especially important when using water-based slurries) and CO
2 [81][97].
Table 3 presents data on studies of thermal decomposition of mixtures carried out in a laboratory and large scale.
Table 3.: 4.9 wt%, C |
2 |
H |
4 | , 6.4 wt%; C | 2 | H | 6 3 wt%.
|
[37][39]
|
|
Lubricating Oil Wastes (LOW)
|
Pyrolysis
|
Laboratory pyrolysis unit. Reactor is heated by an electrical oven. Feeding rate 0.5 g/min. Experiment time 20 min.
|
600–700 °C
|
Pyrolysis gas composition:
H2: 0.01–0.02 g/kg; CO: 0.03–0.04 g/kg; CO2: 0.04–0.08 g/kg; CH4: 0.35–0.93 g/kg; C2H4: 0.5–1 g/kg; C2H6: 0.25–0.47 g/kg.
Product Yield by Pyrolysis:
char: 0.45–0.6 g/kg; liquids: 3.57–6.04 g/kg; gases: 3.46–5.97 g/kg;
|
[61][63]
|
|
Bamboo residual (BR) and waste lubricating oil (WLO)
|
Pyrolysis
| 0.71 |
|
Pyrolyzer with dual catalytic beds HZSM-5 and MgO.
Fast pyrolysis: heating rate 2000 °C/s.
Particle size: 0.15 μm.
|
500–700 °C
|
|
The temperature of 600 °C was optimal due to the relatively high yields of furans and phenols.
0.7
|
[44
15.74
|
]
67.36
|
[
16.9
|
46
-
|
]
[52][54]
|
|
Pyrolytic carbon black
|
93.5 |
|
Coal water ethanol slurry (CWES) (coal 57 wt%, water 36 wt%, ethanol 7 wt%).
|
|
Gasification
2.84
|
Pilot-scale entrained flow gasifier.
Feeding rate at 20 bar: 96.15 kg/h.
<0.01
|
1100 °C
0.46
|
When ethanol was used in the slurry, an increase was recorded in syngas heating value (by 9%), syngas flow rate (by 38%), syngas production per 1 kg of slurry (by 25%), cold gas efficiency (by 39%) and carbon conversion efficiency (by 15%).
Syngas composition: H2: 34.50 vol%; CO: 29.69 vol%; CO2: 35.33 vol%; CH4: 0.47 vol%.
3.2
|
-
|
-
|
-
|
25
|
26
|
[53][55]
|
|
Textile dyeing sludge
|
|
Textile dyeing sludge (DS) with 20–30 wt% additives (CaO, Ca-bentonite, Kaolin and Fe)
|
15.53
|
3.44
|
16.47
|
2.43
|
1.38
|
1.37
|
Pyrolysis
36.53
|
Two-mode microwave device with 2.45 GHz frequency and the maximum power of 3 kW.
Particle size: <1 mm.
1.35
|
|
450–750 °C
|
Addition of CaO and Fe increased the char yield (in 1.2 times) and H2 contents (in 2.5 times), and decreased CO2 content in the non-condensable gas.
Pyrolysis gas composition:
Without additives: H2: 20–33 vol%; CO: 12–15 vol%; CO260.75
|
: 0–65 vol%; CH 4 : 0–5 vol%.
With additives: H2: 12–62 vol%; CO: 15–20 vol%; CO
5.99
|
2
[54][56]
|
: 45–65 vol%; CH | 4 | : 4–15 vol%. |
| Product Yield by Pyrolysis:
char: 60–80 wt%; liquids: 10–14 wt%; gases: 4–15 wt%
|
[54][56]
|
Waste soot
|
|
Corn starch, clover grass, and corn silage in supercritical water
|
74.6
|
Gasification in supercritical water
1.6
|
Continuous flow reactor
-
|
500–700 °C
0.2
|
Gasification of biomass in supercritical water is highly temperature-dependent. Almost complete conversion of the feed can be achieved at 700 °C. As the temperature rises, the H2 yield increases, but the CO concentration decreases.
Syngas composition:
H2: 29.7–34.4 vol%; CO: 0.62–2.8 vol%; CO2: 39.7–43.9 vol%; CH4: 15–20.5 vol%; C2H2: 2.6–4.8 vol%.
1.35
|
68.6
|
-
|
-
|
-
|
28.1
|
[55 |
][57]
|
|
Sewage sludge
|
24.83
|
3.31
|
14.39
|
4.47
|
|
Water–semicoke slurry (semicoke 10–30 wt%).
|
| 1.13 |
|
| 97.95 |
|
Gasification in supercritical water
|
Supercritical water fluidized bed reactor system.
Pressure 23 MPa.
Water flow rate 40 g/min, slurry flow rate 20 g/min/
Particle size: <100 μm
42.74
|
5.39
|
44.58
|
540–660 °C
|
The temperature of 600 °C is the most preferred to provide full gasification of the fixed carbon is realized. The use of K2CO3 as a catalyst made it possible to increase the hydrogen yield by 92%.
Syngas composition:
H2: 50–55 vol%; CO: 2–3 vol%; CO2: 35–38 vol%; CH4
0.77
|
: 10–12 vol%.
[56][58]
|
|
| [ | 46 |
| [ | 52][54] |
Sewage sludge
|
13.22
|
2.91
|
19.7
|
2.12
|
0.57
|
5.29
|
31.31
|
2.06
|
61.34
|
5.215
|
[57][59]
|
|
Coking sludge
|
24.48
|
3.15
|
23.68
|
2.36
|
0.94
|
78.97
|
45.48
|
9.14
|
45.38
|
8.49
|
[58][60]
|
|
Brewery wastewater sludge
|
17.6
|
2.93
|
-
|
2.41
|
-
|
2.33
|
37.72
|
0.09
|
59.86
|
6.56
|
[59][61]
|
|
|
Waste lubricating oil
|
83.53
|
13.32
|
2.83
|
0.15
|
0.17
|
-
|
-
|
-
|
-
|
-
|
[60][62]
|
|
Mineral waste oil
|
83.2
|
13.0
|
1.2
|
-
|
1.2
|
-
|
-
|
|
-
|
-
|
[37][39]
|
|
Lubricating Oil Wastes
|
83.2
|
13
|
1.2
|
-
|
1.2
|
-
|
-
|
-
|
-
|
44.33
|
Based on the literature analysis,
we tcan identify the main research directions on the pyrolysis and gasification of mixed waste-derived fuels
can be identified: (i) pyrolysis and gasification of coal–water slurries with industrial waste additives; (ii) the effect of external conditions on the characteristics of the end products of pyrolysis and gasification; (iii) the use of specialized additives and catalysts to increase the pyrolysis and gasification efficiency.