1. Introduction
The increasing use of microalgae in the cosmetic industry as extraordinary rich source of novel high-value functional products, obtained in eco-friendly and cost-effective processes, is widely recognized
[1][2][1,2]. To date, more than 15,000 novel compounds of algal origin have been identified
[3]. Many bio-based microalgal products are often “multipurposed” and are applied in dermal cosmetics as sunscreens, skin sensitizers and colorants, as well as agents for moisturizing, water-binding, texturizing, thickening, tanning, whitening, etc.
[2][4][5][6][7][8][2,4,5,6,7,8]. In such skin-related applications, the chemically and functionally diverse group of lipids and their derivates comprise a significant gradient
[9][10][9,10]. Apart from the fact that deficiencies in cutaneous lipids cause discomfort, which may lead to serious skin diseases (e.g., atopic dermatitis, psoriasis, acne, rosacea, hereditary ichthyoses, allergic and irritant contact dermatitis and hidradenitis suppurativa), the broad usage of lipids is based also on their ability to form a protective multifunction skin barrier and on their role as emollients or emulsifiers in the bulk of care and make-up products as well
[9][11][9,11]. Today, with the flourishing development of nanotechnology, which leads to a fast product innovation
[12][13][12,13], the potential of lipid nanoparticles (LNP), which seem to be both effective and economic, has been recognized as promising
[14]. For example, in dermal cosmetics, about a decade ago, it was already demonstrated that nano-sized sunscreen products have better performance than micron-sized materials
[15].
Therefore, currently, nano-sized systems are being increasingly studied and applied to encapsulate active ingredients in order to enhance the efficacy of their percutaneous delivery to targeted cells and are also used to improve the physiochemical stability of skin-based cosmetic products
[16]. Such loaded LNP have the advantage of a cumulative effect achieved by a combination of their easier penetration with the enhanced and prolonged release of the carried ingredient to the targeted cellular and subcellular regions
[17][18][19][17,18,19]. In this way, the overall functionality of the final product is improved, allowing LNP-based cosmetic formulations to be highly effective in skin protection, in treating dermatological disorders and in antiaging therapy
[17][18][19][17,18,19]. In addition, many loaded LNP may provide glowing skin
[19]. All these positive effects can be achieved not only by topical skin administrations but also by oral applications
[19], and it is easily explainable that the modern dermal cosmetic industry is searching for new high-value functional lipid products of natural origins.
Moreover, in the recent conditions of our society, with growing consumer demands for vegetal oils, there is a rapidly increasing general interest in lipid-rich microalgae (>20% of lipids on a cell dry weight basis), named oleaginous
[10][20][10,20]. Evidence has been accumulated that the average lipid content in microalgae varies between 1 and 70%, but in certain conditions, it can reach 90% of dry weight (for details, see Reference
[21]). This commercial interest is strongly supported by the fact that many oleaginous microalgae can be cultivated at a production scale both autotrophically and heterotrophically, mostly with inexpensive nutrient regimes, and have faster growth rates with high biomass productivity as compared to terrestrial crops
[2][22][23][24][2,22,23,24]. Further comparisons show that microalgae have even more advantages, since they can be grown all-year-round without the use of arable land, have low water consumption and have low environmental impact
[25]. Another important fact that has to be taken into account is the great general biodiversity of microalgae, which is far away from being thoroughly studied and is gaining increasing attention due to recent surge in searching for indigenous commercially important strains or phycoprospecting
[2][26][27][2,26,27].
Lipids produced by microalgae belong to two major groups of polar lipids and nonpolar, or neutral lipids
[25][28][29][25,28,29]. Polar lipids have an important role in cell structure and cell signaling processes and are commonly known also as structural or membrane lipids
[28][29][28,29]. They comprise a small part of the total lipid fraction in cells (ca. 20%) and usually have long chains of extractable fatty acids (FtAs)
[25][28][29][30][25,28,29,30]. Nonpolar lipids have diverse biological functions, but most of them, triglycerides in particular, are often pointed out as responsible for energy storage
[28]. Most oleaginous microalgae have the capacity to produce significant amounts of nonpolar lipids (up to 80% of the total cell lipid content), the accumulation of which can be influenced, especially in conditions of a lack of nutrients or in stress environments
[25][28][25,28].
The capacity of microalgae to produce lipids in such appreciable amounts, combined with the advantages of their growing, has stimulated considerable interest in their screening for useful and unusual lipid compositions and their mass cultivation as a feedstock for various biotechnological products
[29]. Until now, most research has been oriented towards freshwater, marine and hyperhaline aquatic species from the genera
Aphanizomenon,
Arthrospira,
Chlorella,
Desmodesmus,
Dunaliella,
Haematococcus,
Nannochloropsis,
Scenedesmus and
Spirulina [2]. However, the commercial potential of microalgae inhabiting many other aeroterrestrial or extreme habitats remains untapped
[2]. The species from these inimical environments had to develop ultrastructural, physiological and biochemical adaptive features, which include a series of protective natural compounds of special interest for future applications in human life and cosmetics in particular
[2]. Moreover, such microalgae can be successfully cultivated in outdoor conditions detrimental for standard crops and other algae
[31].
2. Current Insights
The conducted analysis of publications on lipids from AEM issued in the last 56 years showed that most studies addressed PR and FA, specifically carotenoids and FtAs (mainly PUFA), but even these two lipid categories and their accumulation in AEM deserve more attention. In fact, from the eight major groups accepted in LIPID MAPS classification
[32][33][33,34], only five (i.e., FA, GL, GP, ST, PR and PK) have been examined in different phyla of AEM, as follows: Cyanoprokaryota (FA, GL, ST and PR); Rhodophyta (FA, GL, ST, PR and PK); Ochrophyta (FA, GL and PR); Chlorophyta (FA, GL, GP, ST, PR and PK) and Streptophyta (PK)—
Table 1. However, according to the data obtained, it is possible to state that stronger efforts are necessary to improve our knowledge regarding GL, GP and SL and their spread in AEM. Special attention has to be paid to SP, which, according to our best knowledge, remain unknown in AEM.
Table 1.
Main lipid classes investigated in aeroterrestrial (AET) and extremophilic (EXT) microalgae of different taxonomic phyla and classes. Abbreviations: AA—arachidonic acid, ASX—astaxanthin, DGD—digalactosylglycerol, DGTA—diacylglyceryl-hydroxymethyl-N
,N
,N
-trimethyl-β-Ala, DGTS—diacylglyceryl-N
,N
,N-trimethylhomo-Ser, EPA—eicosapentaenoic acid, FA—fatty acyls, FtAs—fatty acids, FAEs—fatty acid esters, GL—glycerolipids, GP—glycerophospholipids, MGD—monogalactosylglycerol, PC—phosphatidylcholine/lecithin, PE- phosphatidyletanolamine/cephalin, PG—phosphatidylglycerol, PI—phosphatidylinositol, PK—polyketides, PR—prenol lipids, PS—phosphatidylserine, PSD—phosphatidylglycerol, SQD—sulphoquinovosyl diacylglycerol, ST—sterol lipids, TGD—triagalactosylglycerol. In Bold are outlined AEM, noted in the cited literature as promising for commercial production and relevant lipids.
| Taxonomic Group/Alga |
Ecological Group |
Investigated Lipid Classes with Examples of Detected Lipids |
References |
| CYANOPROKARYOTA |
|
|
|
| Anabaena cylindrica |
AET |
FA (PUFA—linoleic and linolenic acids, SAFA—palmitic acid and MUFA); GL; ST |
[34] |
| Anabaena cylindrica 1403-2 |
AET |
GL (MGD, DGD, SQD and PSD) |
[35] |
| Anabaena vaginicola |
AET |
PR (lycopene, lutein, beta-carotene, zeaxanthin) |
[36] |
| Calothrix sp. |
AET |
ST |
[34][37][38][39][40] |
| Desmonostoc muscorum |
AET |
FA (PUFA—hexadecadienoic and linoleic acids, SAFA—palmitic acid, MUFA—oleic and palmitoleic acids); GL; ST |
[34][37][38][39][40] |
| Drouetiella lurida |
AET—soil, subaerial |
ST (seven unsaturated ST) |
[41] |
| Microcoleus autumnalis |
AET—soil, subaerial |
ST (cholesterol, β-sitosterol and stigmasterol with squalene as a precursor; ergosterol) |
[40] |
| Nostoc calcarea |
AET—soil, subaerial |
PR (lycopene, lutein, beta-carotene, zeaxanthin) |
[36] |
| Nostoc calcicola B 1459-2 |
AET—soil, subaerial |
FA (PUFA—linolenic acid, SAFA, MUFA), GL—MGD, DGD, SQD and PSD |
[35] |
| Nostoc carneum |
AET—soil, subaerial |
ST |
[34][37][38][39][40] |
| “Nostoc canina” |
AET
(symbiont?) |
FA (PUFA— linoleic acid, SAFA -palmitic acid, MUFA—palmitoleic and oleic acids); GL; ST (cholesterol and lanosterol) |
[34] |
| Nostoc commune |
AET |
ST |
[34][37][38][39][40] |
| Nostoc commune var. sphaeroides |
AET |
ST (campesterol, sitosterol and clionasterol) |
[42][39][43] |
| Nostoc punctiforme PCC73102 |
AET |
FA (FAEs—oxylipins), PR (genes for ASX and canthaxanthin) |
[44][45] |
| Nostoc sp. PCC7120 |
AET |
FA (FAEs—oxylipins) |
[46] |
| Oscillatoria chalybea B1459-2 |
AET |
GL (MGD, DGD, TGD, SQD and PSD) |
|
| Oscillatoria sp. PBGA3 |
AET—soil |
FA (FtAs) |
[47] |
| Scytonema sp. |
AET |
ST (cholest-5-en-3β-ol (18.9 %), 3β-methoxycholest-5-ene (16.2 %) and 3β-acetoxycholest-5-ene (11.2 %), ergosta-5,7,22,24(28)-tetraen-3β-ol) |
[48] |
| Tolypothrix tenuis B1482-3 |
AET |
GL (MGD, DGD, TGD, SQD and PSD) |
[35] |
| Tolypothrix sp. PBGA1 |
AET |
FA (FtAs) |
[47] |
| Tolypothrix sp. PBGA2 |
AET |
FA (FtAs) |
[47] |
| RHODOPHYTA |
|
|
|
| Cyanidium caldarium |
EXT—thermal springs |
GL; ST (ergosta-5,7,22,24(28)-tetraen-3β-ol) |
[49] |
| Cyanidioschyzon merolae |
EXT—thermal springs |
PK |
[50] |
| Galdieria sulfuraria (>47 strains) |
EXT—thermal springs/AET—cryptoendolith |
FA (PUFA—linoleic and linolenic acid, SAFA—palmitic acid, MUFA—oleic and palmitoleic acids); GL (MGD, DGD and SQD; PG, PC, PE, PI, PS and phosphatidate); GP; ST (ergosta-5,7,22,24(28)-tetraen-3β-ol and ergosterol); PR (ß-carotene, lutein); PK |
[51][52][53][49][50] |
| Galdieria sulfuraria/Galdieria sp. |
EXT—acidic but non-thermophilic |
FA (PUFA—linoleic and linolenic acids, SAFA—palmitic, myristic and stearic acids, MUFA—oleic and palmitoleic acids) |
[51][52] |
| Galdieria sp. USB-GBX-832 |
EXT—thermal springs |
FA (PUFA—linoleic acid, AA and EPA; SAFA—palmitic and stearic acid, MUFA—oleic acid) |
[54] |
| Pophyridium purpureum |
AET-soil |
FA (PUFA—AA and EPA); GL |
[55] |
| OCHROPHYTA |
|
|
|
| Eustigmatophyceae |
| Monodopsis subterraneus |
AET—soil |
FA (PUFA—EPA), GL (DGD) |
[56][57][58][59][60][61] |
| Monodus guttula |
AET |
PR (tocopherols) |
[2] |
| Monodus sp. |
AET |
PR (carotenoids—ASX, beta-carotene and lutein) |
[62] |
| Vischeria/Eustigmatos |
AET—soil, subaerial |
PR (total carotenoids; ASX, beta-carotene, lutein and canthaxanthin) |
[62] |
| Tribophyceae (=Xanthophyceae) |
|
|
|
| Botrydiopsis interdecens |
AET |
PR (tocopherols) |
[2] |
| Heterococcus sp. |
AET |
PR (tocopherols) |
[2] |
| Xanthonema sp. |
AET |
PR (tocopherols) |
[2] |
| CHLOROPHYTA |
|
|
|
| Acutodesmus dissociatus TGA1 |
AET—soil |
FA (SAFA—palmitic acid and MUFA—oleic acid) |
[47] |
| Auxenochlorella protothecoides |
AET/EXT—acidic |
PR (carotenoids—lutein) |
[63][64][65][66][67][68][69] |
| Auxenochlorella pyrenoidosa |
AET |
PR (carotenoids—ASX, zeaxanthin, canthaxanthin, lutein) |
[70][71][72][73] |
| Bracteacoccus sp. |
AET |
PR (tocopherols) |
[2] |
| Chlamydocapsa sp. |
EXT—snow |
PR (canthaxanthin, tocopherols) |
[2][74] |
| Chlamydomonas nivalis |
EXT—snow |
PR (ASX, canthaxanthin) |
[74][75][76] |
| Chlamydomonas reinhardtii |
AET |
FA (hydrocarbons—C17 alkene n-heptadecene), GL (betaine lipids—DGTS); GP; ST (ergosterol); PK |
[77][78][79][50][80][81] |
| Chlainomonas sp. |
EXT—snow |
PR (ASX) |
[82] |
| Chlorella sorokiniana |
AET |
ST (ergosterol) |
[79] |
| Chlorella variabilis |
AET |
PK |
[50][80][81] |
| Chlorella variabilis NC64A |
AET (symbiotic) |
ST (ergosterol) |
[79] |
| Chlorella vulgaris |
AET |
FA (free FtAs, FAEs—lactones; hydrocarbons—NC64A eptadecane pentadecane, as well as 7- and 8-heptadecene); GL; ST (ergosterol, 7-dehydroporiferasterol, ergosterol peroxide, 7-dehydroporiferasterol per-oxide and 7-oxocholesterol); PR (carotenoids—ASX, zeaxanthin, canthaxanthin and lutein), PK |
[21][24][83][84][77][85][70][71][72][73] |
| Chlorella sp. PGA2 |
AET—soil |
FA (SAFA, MUFA) |
[47] |
| Chlorella sp. TGA2 |
AET—soil |
FA (SAFA- palmitic acid, MUFA—oleic acid) |
[47] |
| Chlorella sp. TGA4 |
AET—soil |
FA (SAFA, MUFA) |
[47] |
| Chlorococcum sp. (1) |
AET |
PR (carotenoids—ASX (in a free form and as esters), adonixanthin (in a free form and as esters), lutein, canthaxanthin and β-carotene) |
[86][87][88][89][90][91][92] |
| Chlorococcum sp. MA-1 |
AET |
PR (total carotenoids; ASX, lutein, canthaxanthin and ß-carotene) |
[88] |
| Chlorococcum spp. |
EXT—snow |
PR (ß-carotene, lutein and canthaxanthin) |
[51][93][74][75][76][94][95] |
| Chloroidium ellipsoideum |
AET |
PR (carotenoids—zeaxanthin) |
[96] |
| Chloromonas alpina |
EXT—snow |
FA (PUFA, SAFA, MUFA), PR (ASX) |
[51][93][97][98][75][76][94][95] |
| Chloromonas hindakii |
EXT—snow |
FA (PUFA—α-linolenic, stereadonic and hexadecatetraenoic acids, SAFA—palmitic acid and MUFA—oleic acid); GP; PR (ASX) |
[51][93][97][98][75][76][94][95] |
| Chloromonas nivalis |
EXT—snow |
FA (PUFA—hexadecatetraenoic, SAFA and MUFA); PR (ASX, canthaxanthin) |
[51][93][97][98][74][75][76][94][95] |
| Chloromonas nivalis subsp. tatrae |
EXT—snow |
FA (PUFA, SAFA and MUFA); PR (ASX) |
[98] |
| Chloromonas polyptera |
EXT—snow |
FA (PUFA, SAFA and MUFA), PR (ASX) |
[51][93][97][98][75][76][94][95] |
| Chloromonas remiasii CCCryo 005–99 |
EXT—snow |
FA (PUFA-hexadecatetraenoic acid, SAFA and MUFA), PR |
[99][51][93][97][98][75][76][94][95] |
| Chloromonas spp. |
EXT—snow |
FA (PUFA, SAFA—palmitic acid and MUFA—oleic acid), PR |
[93][97][98][75][76][94][95] |
| Chromochloris zofingiensis |
AET |
PR (carotenoids—ASX, canthaxanthin, zeaxanthin, lutein and β-carotene) |
[70][86][87][100][101][102][103][104][105][106][107][108][109] |
| Coccomyxa acidophila |
EXT—acidic |
PR (carotenoids—ß-carotene and lutein) |
[110] |
| Coccomyxa subellipsoidea |
AET |
ST (phytosterols) |
[79] |
| Coccomyxa subellipsoidea C-169 |
AET |
PK |
[50][80][81] |
| Coccomyxa sp. |
AET |
PR (tocopherols) |
[2] |
| Coelastrella oocystiformis |
AET |
PR (carotenoids—ASX esters and canthaxanthin) |
[101][109] |
| Coelastrella striolata var. multistriolata |
AET—subaerial, soils |
FA (PUFA—linoleic acid, SAFA—palmitic acid and MUFA—oleic acid); PR (carotenoids—canthaxanthin, ASX and ß-carotene) |
[111][112] |
| Dunaliella acidophila |
EXT-acidic |
FA (PUFA—linolenic, γ-linolenic and linoleic acids; SAFA; MUFA—oleic and elaidic acids; FAEs—lactones, methyl (12R)-hydroxyoctadeca-9Z,13E,15Z-trienoate, methyl (9S)-hydroxyoctadeca-10E, 12Z,15Z-trienoate and methyl ricinoleate; triacylglycerols—trilinolenin, triolein, trielaidin and tristearin); ST (β-sitosterol, isofucosterol, 24-methylenlophenol, (24S)-methyllophenol and two unidentified sterols, acylsterols and phytol); PR (lycopene, alpha-, beta and gamma-carotene) |
[113][77] |
| Edaphochlamys debaryana |
AET—soil |
FA (FAEs—oxylipins) |
[114] |
| Hindakia tetrachotoma PGA1 |
AET—soil |
FA (SAFA—palmitic acid and MUFA—oleic acid) |
[47] |
| Monoraphidum sp. |
EXT—ice |
FA (PUFA) |
[31] |
| Muriella terrestris |
AET |
PR (tocopherols) |
[2] |
| Muriellopsis sp. |
AET |
PR (carotenoids—lutein) |
[115][116][117][118][119] |
| Neochloris wimmeri |
AET |
PR (carotenoids—ASX esters and canthaxanthin) |
[101][109] |
| Parietochloris alveolaris |
AET-—oil, symbiont |
FA (PUFA—EPA, AA and its precursor dihomo-γ-linolenic acid) |
[120][121] |
| Parietochloris alveolaris K-1 |
AET |
FA (PUFA—α-linolenic acid and EPA) |
[122][123] |
| Protosiphon botryoides |
AET—soil |
PR (carotenoids—ASX esters and canthaxanthin) |
[101][109] |
| Pseudochoricystis ellipsoidea MBIC11204 |
EXT—thermal springs |
FA (FtAs and FAEs—hydrocarbons and triacylglycerols) |
[124] |
| Raphidonema sempervirens |
EXT—snow |
FA (PUFA, SAFA and MUFA); PR (ß-carotene, ASX, lutein and tocopherols) |
[2][93][97][98][74] |
| Sanguina aurantia |
EXT—snow |
PR (ASX) |
[125] |
| Sanguina nivalis |
EXT—snow |
PR (ASX) |
[125] |
| Scenedesmus vacuolatus |
AET |
PR (carotenoids—ASX esters and canthaxanthin) |
[101][109] |
| Scenedesmus spp. |
AET |
PR (total carotenoids, ASX and lutein) |
[112][126][127] |
| Stichococcus bacillaris |
AET |
PR (tocopherols) |
[2] |
| Tetracystis sp. |
AET/EXT—cryotolerant |
PR (canthaxanthin) |
[128] |
| Tetradesmus obliquus |
AET |
FA (PUFA—linolenic, linoleic and linolelaidic acids and SAFA—oleic acid); PR (carotenoids—ASX and lutein) |
[21][25][112][129][130][131][132][133][126][127] |
| Tetradesmus obliquus (strain Scenedesmus obliquus SNW-N) |
AET |
PR (lutein) |
[126] |
| Tetradesmus obliquus (strain Scenedesmus obliquus FSP-3) |
AET |
PR (lutein) |
[134] |
| Ulothrix zonata |
EXT—ice |
FA (PUFA) |
[111] |
| “Unidentified Chlamydomonadaceae” |
EXT—snow |
FA (PUFA, SAFA and MUFA); PR (ASX) |
[47] |
| Unidentified “Chlamydomonadales species” TGA3 |
AET—soil, thermotolerant |
FA (SAFA and MUFA) |
[47] |
| Unidentified “Chlamydomonadales species” TGA5 |
AET—soil |
FA (SAFA and MUFA) |
[47] |
| STREPTOPHYTA |
|
|
|
| Klebsormidium flaccidum |
AET |
PK |
[50] |
-trimethylhomo-Ser, EPA—eicosapentaenoic acid, FA—fatty acyls, FtAs—fatty acids, FAEs—fatty acid esters, GL—glycerolipids, GP—glycerophospholipids, MGD—monogalactosylglycerol, PC—phosphatidylcholine/lecithin, PE- phosphatidyletanolamine/cephalin, PG—phosphatidylglycerol, PI—phosphatidylinositol, PK—polyketides, PR—prenol lipids, PS—phosphatidylserine, PSD—phosphatidylglycerol, SQD—sulphoquinovosyl diacylglycerol, ST—sterol lipids, TGD—triagalactosylglycerol. In Bold are outlined AEM, noted in the cited literature as promising for commercial production and relevant lipids.
The taxonomic diversity of the examined AEM, expressed by the number of species, is as follows: Cyanoprokaryota—20, Rhodophyta—6, Ochrophyta—7, Chlorophyta—53 and Streptophyta—1 (Figure 1A and Table 1). Thus, AEM from green evolutionary lineages (Chlorophyta and Streptophyta) are the most investigated, but a strong discrepancy between the studies inside the green lineages is obvious, with only one species from Streptophyta (i.e., Klebsormidium flaccidum) investigated in comparison with 53 species of 34 genera from Chlorophyta. The next most-studied group is represented by prokaryotic blue–green algae, Cyanoprokaryota, from which 20 species of nine genera have been analyzed. The number of species investigated in Rhodophyta and Ochrophyta is significantly lower and almost similar (six and seven, respectively), but more genera have been covered by studies on Ochrophyta (seven) than from Rhodophyta (four). However, much more strains from Rhodophyta have been examined, with more than 47 strains investigated from a single species (i.e., the extremophilic Galdieria sulfuraria). Most of the studied AEM are unicellular (59), followed by filamentous (26) and coenobial algae (2). The highest morphological diversity has been found in the studied chlorophyte algae, while, from Cyanoprokaryota, only filamentous species and, from Rhodophyta, only unicellular species were examined for different lipids.
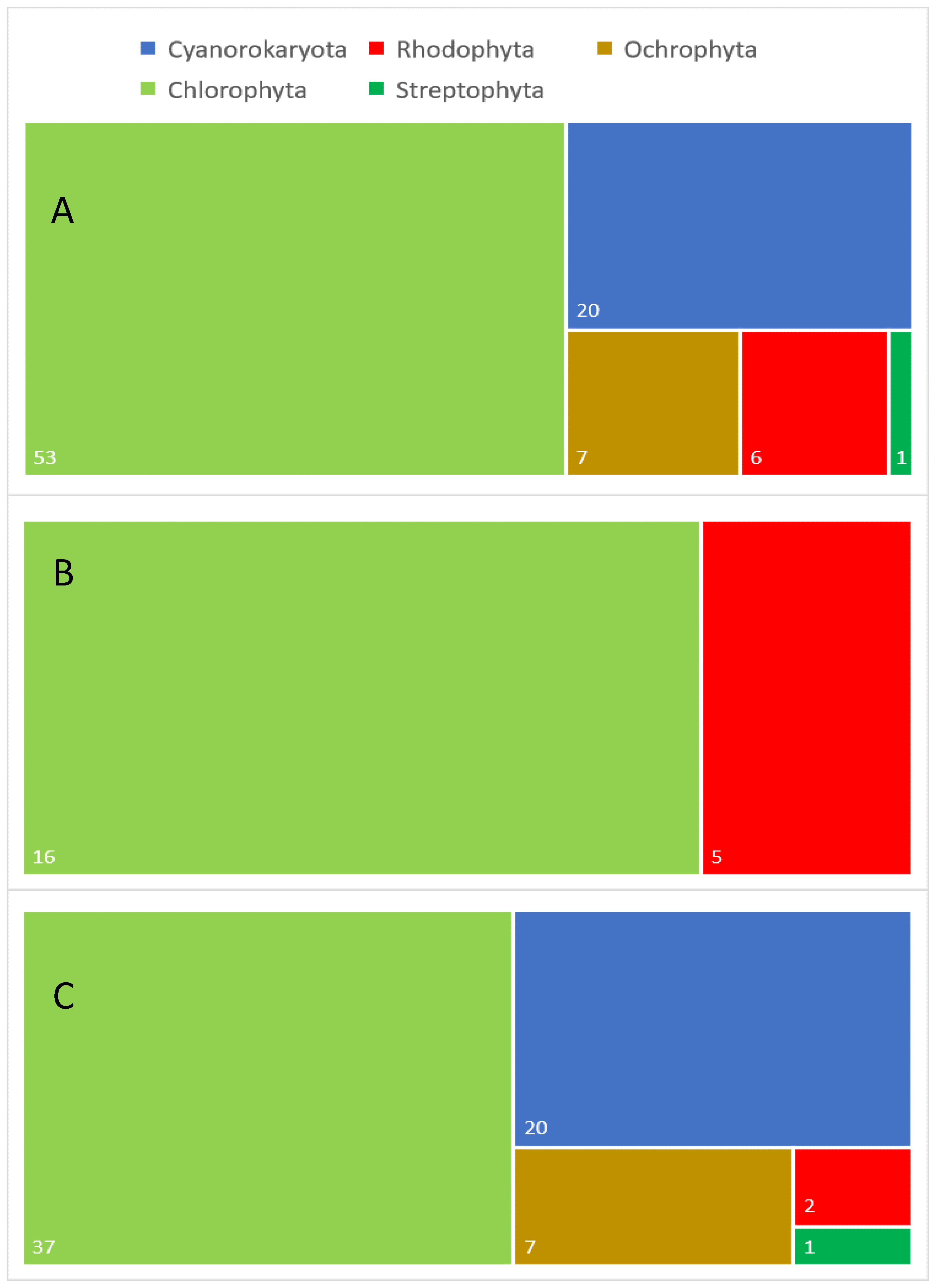
Figure 1.
Taxonomic diversity of aeroterrestrial and extremophilic microalgae analyzed for different lipids: (
A
) general taxonomic diversity of the analyzed algal species, (
B
) taxonomic diversity of the examined extremophilic species and (
C) taxonomic diversity of the investigated aeroterrestrial species. Numbers in white indicate the exact number in each category.
) taxonomic diversity of the investigated aeroterrestrial species. Numbers in white indicate the exact number in each category.
Regarding the ecological affiliation of the examined microalgae (Figure 1B,C), it could be stated that the highest number (66) of studied species are aeroterrestrial, and they are the most taxonomically diverse, including representatives from all five major algal phyla (Figure 1C). By contrast, the number of examined extremophilic species is lower (21), with only two phyla studied (i.e., Rhodophyta and Chlorophyta) (Figure 1B). Despite this generally low number, ten extremophilic algae (ca. 50%) have been considered as promising lipid sources (Table 1). However, it has to be boldly underlined that this analysis is based only on Latin species names provided in the available publications, since, for most of the strains, there are neither data from genetic studies nor detailed morphological and ecological descriptions. In the recent times of rapid taxonomical changes, providing such data in future publications is strongly recommended in order to obtain more precise information on the biochemical compositions of the studied microalgae.
The results obtained clearly showed that very few known AEM have been investigated for their lipid content, with most of the studies being quite scarce and oriented towards certain compounds. Less studies were comparative, but the differences in lipid contents were demonstrated with field materials and cultivated in different conditions algae, or ecologically different strains (e.g., thermophilic and non-thermophilic) were investigated. These results may stimulate further research for the best physiological and cultural conditions, which would lead to the optimal yield of algal biomass and certain lipids. As we have shown in the text above, data on all lipid classes in AEM are far away from being complete, and more investigations on certain compounds and in more AEM are needed in the future. Nevertheless, all collected evidence until now suggests the great potential of AEM as novel commercial lipid sources for versatile cosmetic substances and products for skin care. In this regard AEM are comparable with their aquatic (marine and freshwater) counterparts and land plants, which have been much more intensively studied
[1][2][135][1,2,291]. Twenty-three AEM have been already pointed out as promising for obtaining certain compounds, most of them from Chlorophyta (
Table 1). The potential of AEM as beneficial lipid sources can be recognized in two aspects, separately or in combination: (1) quantitative, since, in some AEM, the contents of valuable lipids are higher in comparison with other algae or plants (e.g.,
Parietochloris alveolaris is considered to be the richest natural source of the high value polyunsaturated ω6 AA, or
Chlorodium ellipsoideum, in which zeaxanthin exceeded more than nine times that of red pepper, a plant source of zeaxanthin) and (2) qualitative, since, in some AEM, rare and unusual lipids were discovered (e.g., the three hydroxy FtAs in
Dunaliella acidophila, such as methyl (12R)-hydroxyoctadeca-9Z,13E,15Z-trienoate, methyl (9S)-hydroxyoctadeca-10E, 12Z,15Z-trienoate and methyl ricinoleate, or the unidentified ST of
Scytonema sp. and
Dunaliella acidophila). These compositional peculiarities in AEM, as it has been shown earlier, are due to their specific ecology and adaptations to survive inimically in other organism environments, and this potential is still untapped
[2]. However, among the AEM examined for lipids occur species from genera that have already been recognized as potential toxin producers or allergic-causative agents
[33][136][34,290]. On that account, in order to answer safety concerns, we strongly underline that all algae, chosen as lipid sources for direct use or for transformation to LNP, must be subjected to chemical analyses before introducing them into mass cultures and cosmetics formulations.