This review summarizes the last scientific findings concerning the use of UV and visible spectrum LED lighting, as ‘Green, sustainable, and low-cost Technologies’, to improve quality of sprouts, microgreens, and baby leaves, to enhance their health-promoting compounds focusing on their mode of action, while reducing costs and energy. These technologies applied either during growing and/or after harvesting have shown to be able to improve physiological, and morphological development of young plants in their first stages of development, while increasing their bioactive compounds content without compromising safety and other quality attributes. The novelty is to summarize the main findings published in a comprehensive review, including the mode of action, and remarking the possibility of its postharvest application where the literature is still scarce.
This entry summarizes the last scientific findings concerning the use of UV and visible spectrum LED lighting, as ‘Green, sustainable, and low-cost Technologies’, to improve quality of sprouts, microgreens, and baby leaves, to enhance their health-promoting compounds focusing on their mode of action, while reducing costs and energy. These technologies applied either during growing and/or after harvesting have shown to be able to improve physiological, and morphological development of young plants in their first stages of development, while increasing their bioactive compounds content without compromising safety and other quality attributes. The novelty is to summarize the main findings published in a comprehensive review, including the mode of action, and remarking the possibility of its postharvest application where the literature is still scarce.
- seed germination
- ultraviolet
- illumination
- light-emitting diodes
- abiotic stress
1. Introduction
2. Elicitor’s Classification: Biotic and Abiotic
3. UV and Visible Spectrum Light Sources
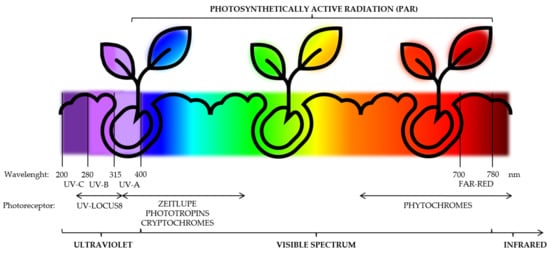
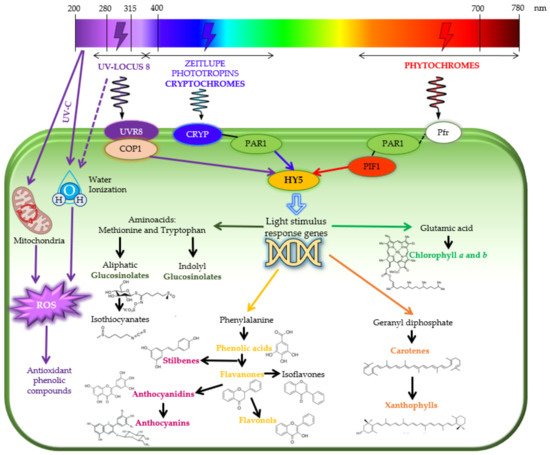
4. Light Stresses as Elicitors of the Biosynthesis of Phytochemicals: Mode of Action
References
- Trichopoulou, A.; Martínez-González, M.A.; Tong, T.Y.N.; Forouhi, N.G.; Khandelwal, S.; Prabhakaran, D.; Mozaffarian, D.; de Lorgeril, M. Definitions and potential health benefits of the Mediterranean diet: Views from experts around the world. BMC Med. 2014, 12, 112.
- Moreira-Rodríguez, M.; Nair, V.; Benavides, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. UVA, UVB light doses and harvesting time differentially tailor glucosinolate and phenolic profiles in broccoli sprouts. Molecules 2017, 22, 1065.
- Abellán, A.; Domínguez-Perles, R.; Moreno, D.A.; García-Viguera, C. Sorting out the value of cruciferous sprouts as sources of bioactive compounds for nutrition and health. Nutrients 2019, 11, 429.
- King, T.; Cole, M.; Farber, J.M.; Eisenbrand, G.; Zabaras, D.; Fox, E.M.; Hill, J.P. Food safety for food security: Relationship between global megatrends and developments in food safety. Trends Food Sci. Technol. 2017, 68, 160–175.
- Infusino, F.; Marazzato, M.; Mancone, M.; Fedele, F.; Mastroianni, C.M.; Severino, P.; Ceccarelli, G.; Santinelli, L.; Cavarretta, E.; Marullo, A.G.M.; et al. Diet Supplementation, Probiotics, and Nutraceuticals in SARS-CoV-2 Infection: A Scoping Review. Nutrients 2020, 12, 1718.
- Pennisi, G.; Blasioli, S.; Cellini, A.; Maia, L.; Crepaldi, A.; Braschi, I.; Spinelli, F.; Nicola, S.; Fernandez, J.A.; Stanghellini, C.; et al. Unraveling the role of red:Blue LED lights on resource use efficiency and nutritional properties of indoor grown sweet basil. Front. Plant Sci. 2019, 10, 305.
- Santin, M.; Ranieri, A.; Castagna, A. Anything new under the sun? An update on modulation of bioactive compounds by different wavelengths in agricultural plants. Plants 2021, 10, 1485.
- Castillejo, N.; Martínez-Zamora, L.; Artés-Hernández, F. Postharvest UV radiation enhanced biosynthesis of flavonoids and carotenes in bell peppers. Postharvest Biol. Technol. 2022, 184, 111774.
- Moreira-Rodríguez, M.; Nair, V.; Benavides, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. UVA, UVB light, and methyl jasmonate, alone or combined, redirect the biosynthesis of glucosinolates, phenolics, carotenoids, and chlorophylls in broccoli sprouts. Int. J. Mol. Sci. 2017, 18, 2330.
- Martínez-Zamora, L.; Castillejo, N.; Artés-Hernández, F. Postharvest UV-B and UV-C radiation enhanced the biosynthesis of glucosinolates and isothiocyanates in Brassicaceae sprouts. Postharvest Biol. Technol. 2021, 181, 111650.
- Castillejo, N.; Martínez-Zamora, L.; Artés–Hernández, F. Periodical Uv-B Radiation Hormesis in Biosynthesis of Kale Sprouts Nutraceuticals. Plant Physiol. Biochem. 2021, 165, 274–285.
- Formica-Oliveira, A.C.; Martínez-Hernández, G.B.; Díaz-López, V.; Artés, F.; Artés-Hernández, F. Effects of UV-B and UV-C combination on phenolic compounds biosynthesis in fresh-cut carrots. Postharvest Biol. Technol. 2017, 127, 99–104.
- Jacobo-Velázquez, D.A.; Cisneros-Zevallos, L. An alternative use of horticultural crops: Stressed plants as biofactories of bioactive phenolic compounds. Agriculture 2012, 2, 259–271.
- Keen, N.T. Specific Elicitors of Plant Phytoalexin Production: Determinants of Race Specificity in Pathogens? Science 1975, 187, 74–75.
- Cisneros-Zevallos, L. The use of controlled postharvest abiotic stresses as a tool for enhancing the nutraceutical content and adding-value of fresh fruits and vegetables. J. Food Sci. 2003, 68, 1560–1565.
- Jacobo-Velázquez, D.A.; Cisneros-Zevallos, L. Bioactive phenolics and polyphenols: Current advances and future trends. Int. J. Mol. Sci. 2020, 21, 6142.
- Jacobo-Velázquez, D.A.; Cisneros-Zevallos, L. An alternative use of horticultural crops: Stressed plants as biofactories of bioactive phenolic compounds. Agriculture 2012, 2, 259–271, Correction in Agriculture 2013, 3, 596–598.
- Surjadinata, B.B.; Cisneros-Zevallos, L. Biosynthesis of phenolic antioxidants in carrot tissue increases with wounding intensity. Food Chem. 2012, 134, 615–624.
- Surjadinata, B.B.; Jacobo-Velázquez, D.A.; Cisneros-Zevallos, L. UVA, UVB and UVC light enhances the biosynthesis of phenolic antioxidants in fresh-cut carrot through a synergistic effect with wounding. Molecules 2017, 22, 668.
- Ortega-Hernández, E.; Nair, V.; Welti-Chanes, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Wounding and UVB light synergistically induce the biosynthesis of phenolic compounds and ascorbic acid in red prickly pears (Opuntia ficus-indica cv. Rojo Vigor). Int. J. Mol. Sci. 2019, 20, 5237.
- Formica-Oliveira, A.C.; Martínez-Hernández, G.B.; Díaz-López, V.; Artés, F.; Artés-Hernández, F. Use of postharvest UV-B and UV-C radiation treatments to revalorize broccoli byproducts and edible florets. Innov. Food Sci. Emerg. Technol. 2017, 43, 77–83.
- Formica-Oliveira, A.C.; Martínez-Hernández, G.B.; Aguayo, E.; Gómez, P.A.; Artés, F.; Artés-Hernández, F. A Functional Smoothie from Carrots with Induced Enhanced Phenolic Content. Food Bioprocess Technol. 2017, 10, 491–502.
- Martínez-Zamora, L.; Castillejo, N.; Artés, F. Postharvest UV-B and Photoperiod with Blue + Red LEDs as Strategies to Stimulate Carotenogenesis in Bell Peppers. Appl. Sci. 2021, 11, 3736.
- Jenkins, G.I. The UV-B photoreceptor UVR8: From structure to physiology. Plant Cell 2014, 26, 21–37.
- Tomás-Callejas, A.; Otón, M.; Artés, F.; Artés-Hernández, F. Combined effect of UV-C pretreatment and high oxygen packaging for keeping the quality of fresh-cut Tatsoi baby leaves. Innov. Food Sci. Emerg. Technol. 2012, 14, 115–121.
- Formica-Oliveira, A.C.; Martínez-Hernández, G.B.; Aguayo, E.; Gómez, P.A.; Artés, F.; Artés-Hernández, F. UV-C and hyperoxia abiotic stresses to improve healthiness of carrots: Study of combined effects. J. Food Sci. Technol. 2016, 53, 3465–3476.
- Rabelo, M.C.; Bang, W.Y.; Nair, V.; Alves, R.E.; Jacobo-Velázquez, D.A.; Sreedharan, S.; de Miranda, M.R.A.; Cisneros-Zevallos, L. UVC light modulates vitamin C and phenolic biosynthesis in acerola fruit: Role of increased mitochondria activity and ROS production. Sci. Rep. 2020, 10, 21972.
- Angelova, Z.; Georgiev, S.; Roos, W. Elicitation of plants. Biotechnol. Biotechnol. Equip. 2006, 20, 72–83.
- Pérez-Balibrea, S.; Moreno, D.A.; García-Viguera, C. Improving the phytochemical composition of broccoli sprouts by elicitation. Food Chem. 2011, 129, 35–44.
- Koutchma, T. Technology of LED Light Sources and Systems from Visible to UV Range. In Ultraviolet LED Technology for Food Applications: From Farms to Kitchens; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 9780128177945.
- Huang, S.-Y.; Lin, J.-C.; Huang, X.-Q.; Wang, W.-K. Large-area 280 nm LED flexible sterilization light source with improved thermal performance. Optik 2021, 248, 168109.
- Náfrádi, M.; Hernadi, K.; Kónya, Z.; Alapi, T. Investigation of the efficiency of BiOI/BiOCl composite photocatalysts using UV, cool and warm white LED light sources—Photon efficiency, toxicity, reusability, matrix effect, and energy consumption. Chemosphere 2021, 280, 130636.
- Jenkins, G.I. Photomorphogenic responses to ultraviolet-B light. Plant Cell Environ. 2017, 40, 2544–2557.
- Taulavuori, K.; Pyysalo, A.; Taulavuori, E.; Julkunen-Tiitto, R. Responses of phenolic acid and flavonoid synthesis to blue and blue-violet light depends on plant species. Environ. Exp. Bot. 2018, 150, 183–187.
- Pennisi, G.; Orsini, F.; Blasioli, S.; Cellini, A.; Crepaldi, A.; Braschi, I.; Spinelli, F.; Nicola, S.; Fernandez, J.A.; Stanghellini, C.; et al. Resource use efficiency of indoor lettuce (Lactuca sativa L.) cultivation as affected by red:blue ratio provided by LED lighting. Sci. Rep. 2019, 9, 14127.
- Bantis, F.; Ouzounis, T.; Radoglou, K. Artificial LED lighting enhances growth characteristics and total phenolic content of Ocimum basilicum, but variably affects transplant success. Sci. Hortic. 2016, 198, 277–283.
- Ohtake, N.; Ishikura, M.; Suzuki, H.; Yamori, W.; Goto, E. Continuous irradiation with alternating red and blue light enhances plant growth while keeping nutritional quality in lettuce. HortScience 2018, 53, 1804–1809.
- Ntagkas, N.; de Vos, R.C.H.; Woltering, E.J.; Nicole, C.C.S.; Labrie, C.; Marcelis, L.F.M. Modulation of the tomato fruit metabolome by LED light. Metabolites 2020, 10, 266.
- Imada, K.; Tanaka, S.; Ibaraki, Y.; Yoshimura, K.; Ito, S. Antifungal effect of 405-nm light on Botrytis cinerea. Lett. Appl. Microbiol. 2014, 59, 670–676.
- Samuoliene, G.; Brazaityte, A.; Viršile, A.; Sirtautas, R.; Sakalauskaite, J.; Sakalauskiene, S.; Duchovskis, P. Photomorphogenetic effects in different plant life forms. Acta Hortic. 2015, 1099, 129–136.
- Park, Y.; Runkle, E.S. Blue radiation attenuates the effects of the red to far-red ratio on extension growth but not on flowering. Environ. Exp. Bot. 2019, 168, 103871.
- Kalaitzoglou, P.; van Ieperen, W.; Harbinson, J.; van der Meer, M.; Martinakos, S.; Weerheim, K.; Nicole, C.C.S.; Marcelis, L.F.M. Effects of continuous or end-of-day far-red light on tomato plant growth, morphology, light absorption, and fruit production. Front. Plant Sci. 2019, 10, 322.
- Park, Y.; Runkle, E.S. Far-red radiation promotes growth of seedlings by increasing leaf expansion and whole-plant net assimilation. Environ. Exp. Bot. 2017, 136, 41–49.
- Jenkins, G.I. Signal transduction in responses to UV-B radiation. Annu. Rev. Plant Biol. 2009, 60, 407–431.
- Meng, Q.; Runkle, E.S. Regulation of flowering by green light depends on its photon flux density and involves cryptochromes. Physiol. Plant. 2019, 166, 762–771.
- Chen, M.; Chory, J.; Fankhauser, C. Light signal transduction in higher plants. Annu. Rev. Genet. 2004, 38, 88–117.
- Chen, M.; Chory, J. Phytochrome signaling mechanisms and the control of plant development. Trends Cell Biol. 2011, 21, 664–671.
- Tian, S.L.; Li, L.; Shah, S.N.M.; Gong, Z.H. The relationship between red fruit colour formation and key genes of capsanthin biosynthesis pathway in Capsicum annuum. Biol. Plant. 2015, 59, 507–513.
- Llorente, B.; Martinez-Garcia, J.F.; Stange, C.; Rodriguez-Concepcion, M. Illuminating colors: Regulation of carotenoid biosynthesis and accumulation by light. Curr. Opin. Plant Biol. 2017, 37, 49–55.
