Serbian goat cheese, quality relies on the use of milk collected from animals reared in organic farming systems. This organic milk contains more dry matter and nutrients; hence, its usage leads to the obtention of dairy products with exceptional nutritional and functional properties. Goat’s milk harbors a specific taste, and it is known to cause less allergic reactions than cow’s milk. The absence of adverse reactions is due to its low or minimal level of the αs1-casein fraction. Goat’s milk is generally more easily digested (the fat globules are smaller) and represents a good source of calcium, phosphorus and vitamins.
- traditional goat cheese
- ripening
- physico-chemical characteristics
- sensory properties
- antibiotic resistance
- safety
1. Introduction
The standard chemical composition of organic and conventional goat milk has been the subject of numerous studies and thereby obtained highly opposing results[1]. Malissiove et al.[2] found no significant differences. On the contrary, Tudisco et al.[3] recorded higher fat content in organic milk, while Pajor et al.[4] apart from the fat, found higher protein content and non-fat dry matter in organic goat milk. Artisan goat cheese with a geographical origin is often produced from raw milk in the traditional way and has its own specific flavor, often robust and very complex, that makes them different from cow milk cheeses. Also, artisanal cheeses process a unique microbiological community.[1][2]
2. Physico-Chemical Analysis
| Goat Milk | Whey | ||
|---|---|---|---|
| Chemical characteristics | Dry matter content (%) | 14.21 ± 0.13 | 7.7 ± 0.00 |
| Milk fat content according to Gerber (%) |
4.60 ± 0.00 |
4. Microbiological Analysis
4.1. Enumeration, Isolation, and Identification of Enterobacteriaceae
| Sample | Day of Analysis | Total Number of Enterobacteriaceae | Total Number of Aerobic Mesophylic Bacteria | |||
|---|---|---|---|---|---|---|
| Milk a | 0 | 1.44 × 104 | 1.63 × 103 | |||
| 0.4 ± 0.01 | ||||||
| Cheese b | 0 | 9.09 × 104 | 3.6 × 106 | Ash content (%) | 0.77 ± 0.01 | 0.53 ± 0.01 |
| Total protein content (%) | ||||||
| Cheese | 3.58 ± 0.02 | 1.36 ± 0.01 | ||||
| 7 | pH value | 6.52 ± 0.02 | 6.52 ± 0.02 | |||
| Titratable acidity (°SH) | 6.53 ± 0.09 | 6.36 ± 0.04 | ||||
| aw value | 0.945 ± 0.00 | 0.946 ± 0.00 | ||||
| Color parameters (D65) | L* | 82.44 ± 0.09 | 39.22 ± 0.03 | |||
| a* | −3.16 ± 0.01 | −2.92 ± 0.07 | ||||
| b* | 6.85 ± 0.00 | 4.53 ± 0.02 | ||||
| Dominant wavelength (nm) |
568.56 ± 0.02 | 565.17 ± 0.27 |
Data are presented as mean ± SD; nd—data not determined.
| 1.87 × 10 |
| 6 |
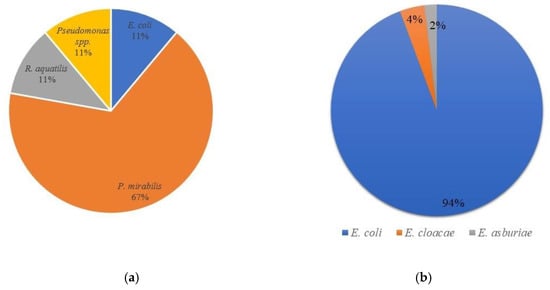
| Species | E. coli | P. mirabilis | R. aquatilis | E. cloacae | E. asburiae | Pseudomonas spp. | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of isolates | 53 | 1 | 6 | 1 | 2 | 1 | 1 | |||||
| Origin (milk or cheese) | cheese | Milk | milk | milk | cheese | cheese | 5.76 × 1010 | |||||
| milk | ||||||||||||
| Biochemical characteristics | Lysine | + | + | − | − | + | + | − | Cheese | 14 | 1.24 × 108 | 5.24 × 1011 |
| Ornitine | − | − | − | − | + | + | + | Cheese | 21 | 3.05 × 107 | 1.20 × 1011 | |
| H2S | − | − | − | − | − | − | + | Cheese | 28 | 1.07 × 105 | 3 × 107 | |
| Glucose | ||||||||||||
| + | ||||||||||||
| + | + | + | + | + | + | |||||||
| Mannitol | + | + | + | + | + | + | − | |||||
| Xylose | + | + | + | + | + | + | + | |||||
| ONPG | + | + | + | + | + | + | − | |||||
| Indole | + | + | − | − | + | − | + | |||||
| Urease | − | − | − | − | + | + | + | |||||
| VP | − | − | + | + | − | + | − | |||||
| Citrate | − | − | + | + | + | + | − | |||||
| TDA | − | − | − | − | − | − | + | |||||
| Gelatin | − | − | − | − | − | − | − | |||||
| Malonate | − | − | − | − | + | − | − | |||||
| Inositol | − | − | − | − | − | − | − | |||||
| Sorbitol | + | + | + | + | + | − | + | |||||
| Rhamnose | + | + | + | + | + | + | + | |||||
| Sucrose | − | − | + | + | + | + | − | |||||
| Lactose | + | + | + | + | + | + | + | |||||
| Arabinose | + | + | + | + | + | + | + | |||||
| Adonitol | − | − | − | − | − | − | − | |||||
| Raffinose | − | − | + | + | + | + | − | |||||
| Salicin | − | − | + | + | + | + | − | |||||
| Arginine | + | + | − | − | + | + | + | |||||
| Growth on citrate medium | Green medium | Green medium | Blue medium |
Blue medium |
Blue medium | Blue medium |
Blue medium |
|||||
| Growth on HiChrome coliform agar | Blue dark/violet | Blue dark/violet | Orange/yellow | Transparent white | Light pink | Light pink | Orange/yellow | |||||
| Microgen GN-A and GN-B |
E.coli | E. coli | Enterobacter amnigenus biogroup 1 | Pantoea agglomerans | Kluyvera ascorbata | Enterobacter gergoviae | Klebsiella oxytoca |
|||||
| MALDI-TOF identification | E. coli | E. coli | P. mirabilis | R. aquatilis | E. cloacae | E. asburiae | Pseudomonas spp. | |||||
| MALDI-TOF score | 2.28 to 2.52 | 2.40 to 2.53 | 2.00 | 2.17 to 2.37 | 2.33 | 1.80 | ||||||
| Proteolytic activity | − | − | − | + | − | − | − | |||||
| Lypolytic activity | − | − | − | − | − | − | − | |||||
| Antibiotic resistance profile | GEN | GEN | AMX, TET | S | AMX, TET | S | AMX, TET | |||||
| Day 0 | Day 7 | Day 14 | Day 21 | Day 28 | ||
|---|---|---|---|---|---|---|
| Chemical characteristics | Dry matter content (%) | 38.91 ± 0.16 a | 41.80 ± 0.11 b | 50.87 ± 0.28 c | 52.64 ± 0.13 d | 46.79 ± 0.06 e |
| Milk fat content according to Van Gulik (%) |
23.00 ± 0.00 a | 25.83 ± 0.23 b | 32.25 ± 0.25 c | 33.5 ± 0.00 d | 32.75 ± 0.25 c,e | |
| Fat in dry matter (%) | 59.11 ± 0.00 a | 61.96 ± 0.00 b | 63.40 ± 0.00 c | 63.64 ± 0.00 c,d | 69.99 ± 0.00 e | |
| Ash content (%) | 2.03 ± 0.17 a | 1.60 ± 0.01 b | 1.66 ± 0.002 b,c | 1.66 ± 0.02 b,c | 1.30 ± 0.01 d | |
| Total protein content (%) | 12.31 ± 0.35 a | 12.54 ± 0.35 a | 14.34 ± 0.14 b | 15.31 ±0.49 b,c | 14.22 ± 0.36 b,c | |
| pH value | 6.55 ± 0.01 a | 5.30 ± 0.01 b | 5.15 ± 0.01 b,c | 4.98 ± 0.00 d | 4.75 ± 0.01 d,e | |
| Titratable acidity (°SH) | 8.53 ±0.37 a | 37.86 ± 0.99 b | 53.60 ± 1.60 c | 61.60 ± 0.80 d | 65.20 ±0.40 e | |
| aw value | 0.941 ± 0.00 a | 0.937 ± 0.00 a | 0.931 ± 0.00 a | 0.929 ± 0.00 a | 0.939 ± 0.00 a | |
| NaCl content (%) | 0.72 ± 0.00 a | 0.80 ± 0.04 b | 0.69 ± 0.01 c | 0.92 ± 0.01 d | 0.96 ± 0.04 d,e | |
| Color parameters (D65) | L* | 88.49 ± 0.69 a | 87.14 ± 0.00 a | 87.95 ± 1.19 a | 87.46 ± 0.77 a | nd |
| a* | −1.88 ± 0.04 a | −1.32 ± 0.44 b | −1.82 ± 0.09 a,c | −1.98 ± 0.16 d | nd | |
| b* | 10.30 ± 0.43 a | 10.95 ± 3.21 a | 12.51 ± 1.11 b | 12.03 ± 0.37 b | nd | |
| Dominant wavelength (nm) |
573.24 ± 0.09 a | 574.27 ± 0.21 a | 573.81 ± 0.11 a | 573.51 ± 0.19 a | nd | |
Data are presented as mean ± SD; nd—data not determined; means within a row marked with different letters differ significantly (p < 0.05).
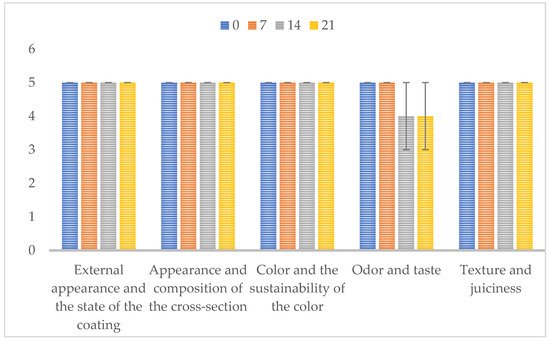
3. Sensory Properties
a CFU/mL of milk, average values of three independent experiments; b CFU/g of cheese, average values of three independent experiments.
“+”—Positive reaction; “−“—Negative reaction; the antibiotic short name whenever the isolate is resistant (GEN—gentamicin; TET—tetracycline; CL—chloramphenicol, AMX—amoxicillin; S—sensitive).
4.2. Proteolytic and Lipolytic Activities
4.3. Antibiotic Resistance Profiles
E. coli O157 Rapid Latex Agglutination Test
Isolation and Identification of Molds
molds. However, mold growth on the cheese surface does not necessarily indicate the presence of mycotoxins in cheese [13]. According to the obtained results, the molds found in cheese samples may indicate poor hygienic conditions during cheese production and/or storage, such as Cladosporium macrocarpum, or from the brine, such as Penicillium aurantiogriseum. Since the toxigenic mold Aspergillus flavus was found only in the cheese sample, after the 21st day of ripening, it is most likely that its occurrence resulted from cross-contamination.[4]
54. Conclusions
References
- Popović-Vranješ, A; Pihler, I; Paskaš, S; Krstović, S; , Jurakić, Ž; Strugar, K.; Production of hard goat cheese and goat whey from organic goat’s milk. Mlijekarstvo 2017, 67, 177–187, https://doi.org/10.15567/mljekarstvo.2017.0302.
- Miloradovic, Z; Tomic, N; Kljajevic, N; Levic, S; Pavlovic, V; Blazic, M; Miocinovic, J; High Heat Treatment of Goat Cheese Milk. The Effect on Sensory Profile, Consumer Acceptance and Microstructure of Cheese.. Foods 2021, 10, 1116, 10.3390/foods10051116..
- Pazzola, M.; Stocco, G.; Dettori, M.; Bittante, G.; Vacca, G.M.; Effect of goat’s milk composition on cheesemaking traits and dairy cheese production.. Journal of Dairy Science 2019, 102, 3947–3955.
- Mladenović, K; Grujović, M; Kocić-Tanackov, S; Bulut, S; Iličić, M; Degenek, J; Semedo-Lemsaddek. T.; Serbian Traditional Goat Cheese: Physico-Chemical, Sensory, Hygienic and Safety Characteristics. Microorganisms 2022, 10, 90, https://doi.org/10.3390/microorganisms10010090.
- Miloradovic, Z.; Miocinovic, J.; Kljajevic, N.; Tomasevic, I.; Pudja, P.; The influence of milk heat treatment on composition, texture, colour and sensory characteristics of cows’ and goats’ Quark-type cheeses. Small Ruminanat Research 2018, 169, 154–159.
- Milovanovic, B.; Tomovic, V.; Djekic, I.; Miocinovic, J.; Solowiej, B.G.; Lorenzo, J.M.; Barba, F.J.; Tomasevic, I.; Colour assessment of milk and milk products using computer vision system and colorimeter.. International Dairy Journal 2021, 120, 105084, https://doi.org/10.1016/j.idairyj.2021.105084.
- Oikonomou, G.; Addis, M.F.; Chassard, C.; Nader-Macias, M.; Grant, I., Delbès; C., Bogni, C.I.; Le Loir, Y.; Even, S.; Milk microbiota: What are we exactly talking about. Frontiers in Microbiology 2020, 11, 60.
- Amorim Angelo, M.B.; dos Santos N.J.; A Highlight for Non-Escherichia coli and Non-Salmonella sp. Enterobacteriaceae in Dairy Foods Contamination. Frontiers in Microbiology 2017, 8, 930.
- Masiello, S.; Martin, N.; Trmčić, A.; Wiedmann, M.; Boor, K.; Masiello, S.; Martin, N.; Trmčić, A.; Wiedmann, M.; Boor, K. Identification and characterization of psychrotolerant coliform bacteria isolated from pasteurized fluid milk.. Journal of Dairy Science 2016, 99, 130–140, https://doi.org/10.3168/jds.2015-9728..
- Baur, C.; Krewinkel, M.; Kranz, B.; von Neubeck, M.; Wenning, M.; Scherer, S.; Stoeckel, M.; Hinrichs, J.; Stressler, T.; Fischer, L.; et al. Quantification of the proteolytic and lipolytic activity of microorganisms isolated from raw milk.. International Dairy Journal 2015, 49, 23–29, https://doi.org/10.1016/j.idairyj.2015.04.005.
- Grujović, M.Ž.; Mladenović, K.G.; Semedo-Lemsaddek, T.; Laranjo, M.; Stefanović, O.D.; Kocić-Tanackov, S.D.; Advantages and disadvantages of non-starter lactic acid bacteria from traditional fermented foods: Potential use as starters or probiotics.. Comprehensive Reviews in Food Science and Food Safety 2022, not known, 1-31, 10.1111/1541-4337.12897.
- Tóth, A.G.; Csabai, I.; Krikó, E.; Tőzsér, D.; Maróti, G.; Patai, Á.V.; Makrai, L.; Szita, G.; Solymosi, N.; Antimicrobial resistance genes in raw milk for human consumption. Scientific Report 2020, 10, 7464..
- Dobson, A.D.W.. Chapter 23-Mycotoxins in Cheese; McSweeney, P.L.H., Fox, P.F., Cotter, P.D., Everett, D.W., Eds.; Academic Press: Elsevier, 2017; pp. 595–601.
- Grujović, M.Ž.; Mladenović, K.G.; Semedo-Lemsaddek, T.; Laranjo, M.; Stefanović, O.D.; Kocić-Tanackov, S.D.; Advantages and disadvantages of non-starter lactic acid bacteria from traditional fermented foods: Potential use as starters or probiotics.. Comprehensive Reviews in Food Science and Food Safety 2022, not known, 1-31, 10.1111/1541-4337.12897.
- Tóth, A.G.; Csabai, I.; Krikó, E.; Tőzsér, D.; Maróti, G.; Patai, Á.V.; Makrai, L.; Szita, G.; Solymosi, N.; Antimicrobial resistance genes in raw milk for human consumption. Scientific Report 2020, 10, 7464..
- Dobson, A.D.W.. Chapter 23-Mycotoxins in Cheese; McSweeney, P.L.H., Fox, P.F., Cotter, P.D., Everett, D.W., Eds.; Academic Press: Elsevier, 2017; pp. 595–601.
