Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Amina Yu and Version 1 by Andrea Palermo.
Concentrated Growth Factors (CGF) represent new autologous (blood-derived biomaterial), attracting growing interest in the field of regenerative medicine.
- CGF
- growth factor
- stem cells
- blood-derived biomaterials
- osteogenic differentiation
1. Introduction
In the field of regenerative medicine, there is growing interest in platelet concentrates derived from whole blood in order to improve tissue regeneration processes.
These preparations contain high concentrations of growth factors, such as platelet-derived growth factor (PDGF), transforming growth factors β1 (TGF-β1) and β2 (TGF-β2), vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), and insulin-like growth factor (IGF), which are all involved in cell proliferation, matrix remodeling and angiogenesis [1].
Platelet derivatives have several medical applications, including stimulation of tissue regeneration in dentistry, implantology and plastic surgery, healing of recalcitrant ulcers and burns, repair of musculoskeletal tissue, tendon and ligament lesions, and osteoarthritis treatment [2]. The versatility of these blood derivatives is linked to their autologous nature and simple collection and preparation methods [2].
Platelet derivatives can be classified into three different generations based on their characteristics and preparation methods.
The first generation, developed in the 1970s, is platelet-rich plasma (PRP). It contains several growth factors implicated in tissue repair, but for the fibrin polymerization induction, the preparation requires the use of anticoagulants and bovine thrombin, which interfere with the natural healing process [3,4][3][4].
The second generation consists of platelet-rich fibrin (PRF). For its preparation, blood samples are collected without using anticoagulants or biological agents. PRF has been further modified into an advanced form called advanced platelet-rich fibrin (A-PRF), which has a fibrin clot softer than PRF and a number of platelet cells greater than PRF [5].
The third and latest generation of platelet derivatives developed by Sacco in 2006 [6] is called concentrated growth factors (CGF), and it can be considered another modified form of PRF. CGF is produced by centrifugation of the blood sample using alternating speed rates. This process leads to a dense fibrin matrix, which can promote the migration of cells, such as fibroblast and endothelial cells [1], and contains more growth factors than PRP and PRF [5,7][5][7]. Furthermore, the presence of CD34-positive stem cells, in addition to leukocytes, has been demonstrated in CGF [1].
Differences in the growth factors released among PRP, PRF, A-PRF, and CGF have been reported. PRF and A-PRF released, in a constant way, a total amount of growth factors higher than PRP, which released most of the growth factors at the beginning of culture [4,8][4][8].
It has been reported that both A-PRF and CGF contain a higher amount of growth factors than PRP and PRF [5]. Moreover, analyzing the releases of some growth factors by CGF in an eight-day period, it has been shown that different growth factors had different release kinetics [9]. Plasma rich growth factors (PRGF) also contain multiple growth factors and cytokines; PRGF-modified collagen membranes allowed the kinetic release of these therapeutic molecules that enhanced bone regeneration and soft tissue healing [10]. However, to date, the growth factors released by CGF in a longer period have not yet been studied.
Some recent findings have opened interesting perspectives on the biotechnological use of CGF in the tissue regeneration field. The CGF-enhanced proliferation of three cultured cell lines (fibroblasts, endothelial cells, and osteoblasts) through the release of growth factors with specific kinetic accumulations, suggesting that a programmed release might support the regeneration process [9]. CGF alone is able to induce osteogenic differentiation of human bone marrow stem cells (BMSC) [11].
Several studies in vivo have stated improvements in tissue healing or regeneration in the presence of CGF [12]. It has also been reported that a better effect in bone formation occurs with CGF than with PRF in femur defects of adult dogs [13]. Moreover, a combination of CGF with stem cells or grafts determined better results than CGF alone [12].
Several authors have also shown that besides growth factors and platelets, the resident and circulating monocytes/macrophages and multipotent stem cells are important in the processes of tissue regeneration and differentiation [14,15][14][15].
Although a growing body of evidence suggests the existence of multipotent cells in peripheral blood [16[16][17],17], to date, the use of blood as an alternative source of autologous stem cells in regenerative medicine is limited by important questions: the predictability of successful isolation and ex vivo expansion by a standardized protocol.
The aim of this work was the chemical, structural, and biological characterization of CGF to deepen the knowledge of this very promising biomaterial in the field of regenerative medicine.
2. CGF: Fibrin and Cellular Components
To evaluate the features of the fibrin network and the cell content of CGF, the external and inner surfaces of its middle part were analyzed by SEM. The two surfaces showed different aspects. As shown in Figure 2a, on the CGF external surface, a dense fibrin network and few corpuscular elements, including activated platelets, were found (Figure 2b). The CGF inner surface presented high activated platelet zones and many cells (Figure 2c,d).
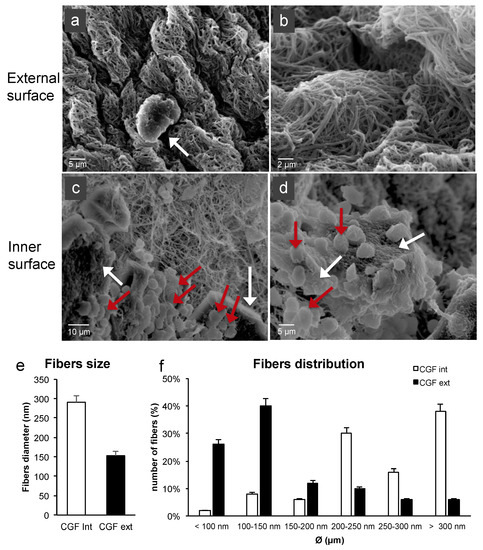

Figure 2. SEM images of fresh CGF. (a) The external surface of CGF was characterized by few activated platelets (white arrow) within the fibrin matrix. (b) Fibrin network appeared densely packed. (c,d) The inner surface of CGF showed a large population of activated platelets (white arrows) and white blood cells (red arrows). (e,f) Average diameters and size distribution of fibrin fibers were calculated using ImageJ software. The results were expressed as the means ± standard deviation (SD) of 50 measurements from each acquired sample.
The CGF fibers of the external surface seemed to be partially fused together. The fiber distribution analysis revealed an average diameter of 291 ± 16 nm and 153 ± 11 nm for the inner and external CGF surfaces, respectively (Figure 2e). Most of the fibers were included in the 100–150 nm range for the external surface and had a diameter larger than 300 nm for the inner surface. The distribution analysis highlighted that most of the fibers were included in the 100–500 nm range, similarly to the extracellular matrix (ECM) nanoarchitectures (Figure 2f).
In order to evaluate cell distribution, density, and morphology in CGF, hematoxylin and eosin staining were carried out. Figure 3 shows images of CGF sections from three different experimental conditions: CGF prepared fresh (0 days), CGM cultured for 14 days, and CGM cultured for 28 days.
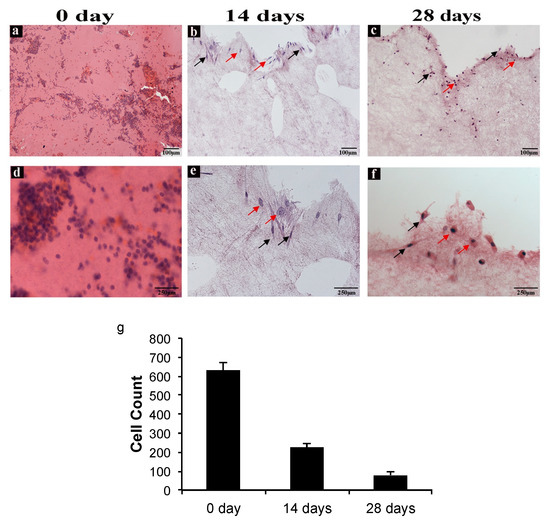

Figure 3. CGF hematoxylin-eosin staining. (a,d) Sections of CGF at time zero (0 day), (b,e) after 14 days, and (c,f) after 28 days of incubation in culture medium. The red arrows indicate the spherical cells, and the black arrows indicate the spindle-shaped cells. (a–c) Scale Bar: 100 μm, (d–f) 250 μm. (g) The number of cells in the CGF sections at 0, 14, and 28 days were calculated using ImageJ software. All values were expressed as mean ± SD (n = 3 per group, 3 replicates).
A reduction of cell density in CGF sections at both 14 and 28 days with respect to that at 0 days was observed (Figure 3a–c) and confirmed through cell counts (Figure 3g). Moreover, at day zero, cell distribution was homogeneous all over the section, whereas at 14 and 28 days, the cells appeared isolated or were forming small groups, especially in the peripheral area of the sections. As shown in Figure 3, there are two morphologically different cell types: spindle-shaped and spherical.
Therefore, CGF cells were immunolabeled with anti-CD34, CD45, and CD105 antibodies directed against cell surface markers, to characterize their immunophenotype. Immunostaining showed CD34+, CD45+, and CD105+ cells (Figure 4). All stainings showed a reduction of immunoreactivity in CGF at 14 and 28 days compared to that at 0 days (Figure 4).
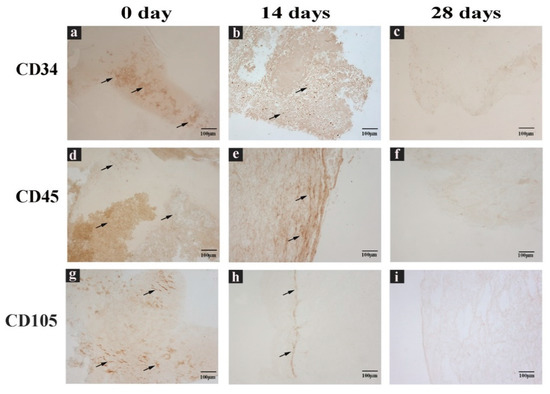

Figure 4. CGF sections labeled with immunohistochemical assay. (a,d,g) Sections of CGF at 0 days, (b,e,h) 14 days, and (c,f,i) 28 days of incubation in culture medium. Incubated with (a–c) anti CD34 antibody, (d–f) anti CD45 antibody, or (g–i) anti CD105 antibody. Black arrows indicate the positive immunolabel. Scale Bar: 100 μm.
3. CGF Cells Display Pluripotency and Stem Cell Markers
After 14 days in the culture medium, CGF released a mixture of cells (Figure 5a). In order to facilitate the release of the cells trapped in the CGF, the latter was chopped. Then the pieces were put into new culture plates where, after 7–10 days, a consistent population of adherent mononuclear cells was observed. Many cells had a spindle-shaped morphology, and few cells were spherical (Figure 5b). CGF adherent cells were able to proliferate, maintaining their own aspect across subsequent passages (Figure 5c,d).
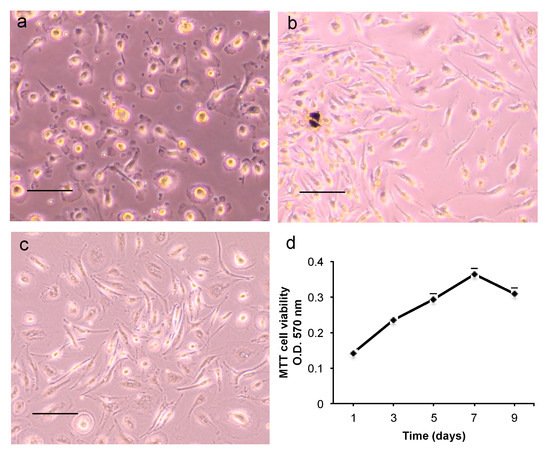

Figure 5. Morphology and cell proliferation of CGF primary cells. (a) Adherent cells released by the whole CGF at 14 days; (b) cells from CGF pieces after 14 days; (c) cells at third passage. Scale bar: 100 μm. (d) CGF primary cell viability was assessed by MTT assay on days 1, 3, 5, 7, 9 after cell seeding. Data represent mean ± SD of duplicate measurements from three independent experiments.
To characterize CGF adherent cells, the expression of mesenchymal and hematopoietic stem cell markers was evaluated by flow cytometry. The analysis of hematopoietic markers showed that 90% of CGF cells expressed CD45. More than 90% expressed mesenchymal stem cell marker CD105, while other markers were not detected (CD90 and CD73) or were expressed at low levels (CD34) (Figure 6).


Figure 6. Flow cytometry analysis of mesenchymal and hematopoietic surface markers. Representative flow cytometry histogram of CGF cells. Grey histogram: isotype control; open histogram: signal for each specific antibody. Values in the table represent the percentage of positive cells for the dye as the mean ± SD.
The expression of cell surface markers and transcription factors required to maintain the pluripotency and self-renewal in stem cells (oct3/4 and Nanog) or to determine hema-topoietic development (Stat4) was analyzed by real-time PCR. CGF adherent cells showed high CD31, CD36, CD105, and CD45 mRNA levels; consistent mRNA levels of CD14, OCT-3, and STAT4 were also found, whereas low CD90, CD73, CD34, and Nanog mRNA levels were detected (Figure 7a).


Figure 7. Gene expression of cell surface and pluripotent markers. (a) mRNA was quantified by real-time PCR in CGF primary cells. The comparative CT method (2−ΔCT ± SD) was used to quantify the gene expression level. Gapdh was used as a housekeeping gene. The results are expressed as the mean ± SD of triplicate measurements from four independent experiments. (b) Expression of stem cell surface proteins. β-Actin was used as an internal loading control. The image is representative of three independent experiments.
To further assess the expression of surface markers in cells released by CGF, a Western blotting analysis was carried out. In agreement with real-time PCR quantitation, CGF cells expressed high CD45, CD14, and CD105 protein levels. CD90 and CD34 protein levels were very weakly detectable (Figure 7b).
4. Characterization of the Stemness Features of CGF Cells and Osteogenic Potential
In recent years CGF was widely studied as an autologous blood derivative able to promote tissue repair, vascularization, cell migration, and differentiation [11,19,20,21,22][11][18][19][20][21]. Tissue repair is a complex mechanism that takes place over four phases: inflammatory process, cell proliferation, differentiation, and ECM remodeling. The process involves cytokines, growth factors, and MMPs [15]. Despite a large literature on CGF use and applications in the regenerative medicine field [21[20][22],23], up to the present, no data are provided on the metabolomic profile of CGF, and very few studies investigated the kinetic release of CGF growth factors and MMPs over a long time and analyzed the CGF cellular component. The aim of this work was to characterize the CGF metabolites composition, the amount of growth factors and MMPs released by CGF over a period of 28 days, and to study in detail the CGF cellular components.
GC/MS metabolomics analysis highlighted the high concentration of L-glutamic acid and taurine in CGF and the statistically different amount of the two analytes between the CGF and PPP fractions. These results are quite interesting considering the CGF application in the field of regenerative medicine. Indeed, it was demonstrated that ECM proteins and biomaterials, functionalized with amino acid sequences rich in glutamic acid, induced osteogenic differentiation, and mineralization of marrow stromal cells [24][23]. In fact, glutamic acid residues are known to act as a nucleation point for calcium phosphate mineralization [25][24]. Furthermore, taurine, a non-essential amino acid, has been shown to have positive effects on bone mass and influence bone metabolism [26][25]. Taurine was also shown to promote the differentiation of human MSC into osteoblasts and to upregulate the expression of osteoblast markers as osterix, Runx2, osteopontin, and alkaline phosphatase via ERK1/2 signaling [27][26]. In a recent study, it weas reported that the ability of CGF to promote the osteoblast differentiation of BMSC [11]. This capacity could be due to the high levels of L-glutamic acid and taurine and to prolong release from CGF of some growth factors, as reported in the present study. In fact, the initial amount of some bioactive molecules extracted from CGF was analyzed soon after preparation, then their release from CGF was quantified over time. WeIt was found that CGF extract contained growth factors such as VEGF, TGF-β1 and BMP-2, and MMPs (such as MMP-2 and MMP-9), confirming previous studies [28,29,30][27][28][29]. Moreover, to mimic the natural release of soluble factors, we cultured CGF, without any manipulation, in cell culture medium, at different times, until 28 days. We found that growth factors and MMPs were gradually released over time up to 28 days from CGF preparation, following specific release kinetics. In particular, VEGF was released slowly up to 14 days, when it reached its maximum value and gradually decreased over time. Similar to VEGF, TGF-β1 and BMP-2 were also released slowly. They peaked at 21 days, and their values remained high up to 28 days. The matrix-degrading enzymes MMP-9 and MMP-2 were released faster than the growth factors and peaked after seven days, with MMP-9 more abundant than MMP-2, then gradually decreased over time. The present findings reported, for the first time, a continuous and prolonged release of multiple bioactive factors over time, suggesting that CGF is suitable in promoting the complex and long process of tissue regeneration. To the best of our knowledge, 28 days is the longest time analyzed for the release of factors from the CGF. Indeed, previous studies analyzed CGF growth factors release within shorter time intervals [9,31,32,33][9][30][31][32].
Two phases in the release of growth factors by CGF have been reported [34][33]: an immediate phase, which could be attributed to the instantaneous release from activated platelets during centrifugation or to simple diffusion; a late phase with accumulation peaks at 14 days, which could be explained by the release of growth factors after degradation of the fibrin structure and by the production of growth factors from the CGF resident cells [21,35,36][20][34][35].
Concordantly, it weas found that the growth factors and MMPs released in the conditioned medium from the CGF reached higher amounts than the initial ones extracted from the CGF, suggesting a role of CGF-resident cells in the synthesis and secretion of these factors. In particular, the amount of VEGF in the CGF-conditioned medium after a 14-day incubation was even greater than the amount of VEGF extracted from the CGF. These results agree with our previous study showing that CGF-derived cells expressed and released angiogenic factors, including VEGF [22][21].
Growth factors are considered essential elements in tissue regeneration; they play a critical role in regulating processes involved in wound healing and tissue repair, so their amounts and release kinetics, as weit found, could be important to better assess the efficacy of CGF.
Among the multiple growth factors released by CGF, VEGF is a crucial molecule in tissue repair and regeneration since it is implicated in angiogenesis, blood vessel growth from pre-existing vasculature and vasculogenesis, and de novo formation of blood vessels [37][36]. VEGF has been demonstrated to stimulate endothelial cell proliferation and promote angiogenesis by binding to a high-affinity receptor, and its signaling is considered a rate-limiting step in the initiation of angiogenesis [38][37]. However, due to the very short half-life of VEGF [39[38][39],40], low efficacy is achieved when administered as free proteins because high doses have a prohibitive cost and often cause undesirable effects [41][40]. Therefore, a sustained release system of VEGF is required to provide ideal therapeutic effects, which could be achieved by CGF application.
In our experimental conditions, TGF-β1 was the most abundant growth factor contained in and released by CGF over time. TGF-β1 is a secreted protein that regulates many cellular functions, including the control of immune and stem cell growth, proliferation, differentiation, apoptosis, development, and tissue remodeling following injury [42,43][41][42]. Thus, the release of TGF-β1 is desirable in wound healing sites and particularly in the oral cavity, where several types of cells, like fibroblasts and osteoblasts, must be stimulated to proliferate. Temporal and spatial activation of TGF-β is involved in the recruitment of stem/progenitor cells and participation in the tissue regeneration/remodeling process. BMP-2, another important member of the TGF-β superfamily, plays a key role in the development of bone and cartilage. It is a highly potent growth factor able to promote the differentiation and maturation of osteoblasts [44][43]. WeIt was found that BMP-2 was the growth factor released by CGF in the lowest amounts. However, BMP-2 has been shown to be released from platelets, mainly at low pH [45][44], which is the common environment of wound healing sites [46][45]. Therefore, the use of CGF could improve the repair processes by locally stimulating the release of BMP-2 at the injury site. Moreover, it weas also found that CGF released the MMP-2 and MMP-9. MMPs are matrix-degrading enzymes implicated in many biological processes, including inflammation and cell migration during wound healing and tissue repair in coordination with several growth factors and cytokines [47][46].
The importance of the resident and circulating cells in the processes of tissue regeneration is well established [14,15][14][15]; therefore, besides growth factors and molecules contained in and released by CGF, wehich focused on the characterization of its cellular components.
SEM observation did not reveal the presence of cells on the surface of CGF but showed a fibrin framework denser than inside of CGF, where large populations of activated platelets and cells were present. Immunohistochemistry analysis of CGF showed a very uniform distribution of nucleated cells entrapped in the fibrin network. The sections reacted positively to CD34, CD45, and CD105 immunolabelling. Indeed, the presence of different cell populations is known: hematopoietic stem cells, lymphocytes, monocytes, and fibroblast-like cells [1]. Our recent findings showed that when CGF, without manipulation, is released into the culture medium, cells are able to adhere to the plate and proliferate [22][21]. Here it we showas shown that the release of cells from CGF seemed to be rather slow, and most of the cells were found in the plate only after cutting CGF on the 14th day. This aspect could be correlated with hematoxylin-eosin staining data and with CGF fibrin network structure observed by SEM analysis: indeed, while at the initial stage CGF cell distribution was homogeneous all over the section, after two and four weeks, cells seemed to migrate from the center where fibrin network was less dense to the peripherical area of the sections, where fibrin appeared to be more densely intertwined. This scenario might explain either why cells were retained into CGF so long (up to 28 days) and the sustained release kinetics of CGF growth factors and MMPs.
Di Liddo et al. recently reported that the leukocyte- and platelet-rich fibrin product called CPL-MB acts as an artificial stem niche containing autologous multipotent cells with defined stemness properties [48][47]. In our work, CGF primary cells showed fibroblast-like and spherical morphology; however, after few passages, cell populations appeared to be enriched in spindle-shaped cells and showed different surface markers with respect to cells resident in the CGF. Indeed, adherent cells expressed a high level of CD105 and CD45 surface markers; whereas, CD34 was scarcely detectable. Since it weas found that CGF primary cells exhibited monocyte markers, such as CD31, CD45, CD14, and CD36, [49,50][48][49] which weas assumed that they might be monocyte-derived cells. The primary CGF cells did not appear as mesenchymal stem cells derived from peripheral blood since they did not express CD73 and CD90 mesenchymal markers; however, they showed mesenchymal, hematopoietic, and endothelial stem cell features. Indeed, it has been demonstrated that monocyte-derived cells expressing CD105, CD45, and CD14 exhibit mesenchymal cell features and are able to differentiate into different cell lines [49][48].
In addition, CGF primary cells express genes that denote the molecular signature of stem cell pluripotency, including Oct3/4 and Nanog. The transcription factor Oct3/4 is thought to be indispensable for pluripotency in stem cells and is expressed in multipotent progenitor cells isolated from peripheral blood [17]. Nanog is a key factor in the self-renewing of embryonic stem cells, which remained pluripotent after multiple passages, but it has a heterogeneous expression mode; indeed, Nanog-negative cells show a higher propensity for differentiation [51][50]. OurThe results show low Nanog mRNA levels. WeIt also reported high STAT4 mRNA levels. STAT4 is a key transcription factor involved in promoting cell-mediated immunity, but its expression is not restricted to lymphoid cells. Activated monocytes expressed STAT4 in response to Interferon-alfa [52][51], a cytokine that downregulates osteoblastogenesis [53][52], though increases the formation of calcific nodules under osteogenic conditions in human aortic valve interstitial cells [54][53].
Finally, to better characterize the use of CGF in the field of regenerative medicine, since CGF primary cells seem to display several pluripotency markers, the ability of these cells to differentiate into osteoblasts was tested. Interestingly, we found that CGF primary cells, kept three weeks in osteogenic medium, were able to differentiate into osteoblasts as demonstrated by the formation of mineralized nodules, the expression of the osteogenic markers RUNX2, COL1a1, and OCN, and the loss of stem cell markers [11]. These results suggest that CGF could also represent a source of cells with stem features, thus expanding its potential applications.
Recently, we demonstrated the ability of CGF to promote the osteogenic differentiation of stem cells [11]. Furthermore, we showed that CGF releases endothelial progenitor cells, which contribute to neo-angiogenesis and to the formation of endothelial tubular structures [22].
Here we reported that CGF has a complex inner structure capable of influencing the release of growth factors, metabolites, and cells. These cells, which could regulate the production and release of the CGF growth factors, show stem features and are able to differentiate into osteoblasts, producing a mineralized matrix. These data, taken together, highlight interesting new perspectives for the use of CGF in tissue regeneration and in regenerative medicine.
References
- Rodella, L.F.; Favero, G.; Boninsegna, R.; Buffoli, B.; Labanca, M.; Scarì, G.; Sacco, L.; Batani, T.; Rezzani, R. Growth factors, CD34 positive cells, and fibrin network analysis in concentrated growth factors fraction. Microsc. Res. Tech. 2011, 74, 772–777.
- Masoudi, E.; Ribas, J.; Kaushik, G.; Leijten, J.; Khademhosseini, A. Platelet-Rich Blood Derivatives for Stem Cell-Based Tissue Engineering and Regeneration. Curr. Stem. Cell Rep. 2016, 2, 33–42.
- Liu, Y.; Sun, X.; Yu, J.; Wang, J.; Zhai, P.; Chen, S.; Liu, M.; Zhou, Y. Platelet-Rich Fibrin as a Bone Graft Material in Oral and Maxillofacial Bone Regeneration: Classification and Summary for Better Application. BioMed Res. Int. 2019, 2019, 1–16.
- Kobayashi, E.; Flückiger, L.; Fujioka-Kobayashi, M.; Sawada, K.; Sculean, A.; Schaller, B.; Miron, R.J. Comparative release of growth factors from PRP, PRF, and advanced-PRF. Clin. Oral. Investig. 2016, 20, 2353–2360.
- Masuki, H.; Okudera, T.; Watanebe, T.; Suzuki, M.; Nishiyama, K.; Okudera, H.; Nakata, K.; Uematsu, K.; Su, C.Y.; Kawase, T. Growth factor and pro-inflammatory cytokine contents in platelet-rich plasma (PRP), plasma rich in growth factors (PRGF), advanced platelet-rich fibrin (A-PRF), and concentrated growth factors (CGF). Int. J. Implant Dent. 2016, 2, 19.
- Sacco, L. Lecture, International Academy of implant prosthesis and osteoconnection. Lecture 2006, 12, 4.
- Sohn, D.S.; Heo, J.U.; Kwak, D.H.; Kim, D.E.; Kim, J.M.; Moon, J.W.; Lee, J.H.; Park, I.S. Bone regeneration in the maxillary sinus using an autologous fibrin-rich block with concentrated growth factors alone. Implant Dent. 2011, 20, 389–395.
- Schär, M.O.; Diaz-Romero, J.; Kohl, S.; Zumstein, M.A.; Nesic, D. Platelet-rich concentrates differentially release growth factors and induce cell migration in vitro. Clin. Orthop. Relat. Res. 2015, 473, 1635–1643.
- Borsani, E.; Bonazza, V.; Buffoli, B.; Cocchi, M.A.; Castrezzati, S.; Scarì, G.; Baldi, F.; Pandini, S.; Licenziati, S.; Parolini, S.; et al. Biological Characterization and In Vitro Effects of Human Concentrated Growth Factor Preparation: An Innovative Approach to Tissue Regeneration. Biol. Med. 2015, 7, 256.
- Ratiu, C.; Brocks, M.; Costea, T.; Moldovan, L.; Cavalu, S. PRGF-Modified Collagen Membranes for Guided Bone Regeneration: Spectroscopic, Microscopic and Nano-Mechanical Investigations. Appl. Sci. 2019, 9, 1035.
- Rochira, A.; Siculella, L.; Damiano, F.; Palermo, A.; Ferrante, F.; Carluccio, M.A.; Calabriso, N.; Giannotti, L.; Stanca, E. Concentrated Growth Factors (CGF) Induce Osteogenic Differentiation in Human Bone Marrow Stem Cells. Biology 2020, 9, 370.
- Tabatabaei, F.; Aghamohammadi, Z.; Tayebi, L. In vitro and in vivo effects of concentrated growth factor on cells and tissues. J. Biomed. Mater. Res. A 2020, 108, 1338–1350.
- Park, H.C.; Kim, S.G.; Oh, J.S.; You, J.S.; Kim, J.S.; Lim, S.C.; Jeong, M.A.; Kim, J.S.; Jung, C.; Kwon, Y.S.; et al. Early Bone Formation at a Femur Defect Using CGF and PRF Grafts in Adult Dogs: A Comparative Study. Implant Dent. 2016, 25, 387–393.
- Anghelina, M.; Krishnan, P.; Moldovan, L.; Moldovan, N.I. Monocytes/macrophages cooperate with progenitor cells during neovascularization and tissue repair. Am. J. Pathol. 2006, 168, 529–541.
- Manole, E.; Niculite, C.; Lambrescu, I.M.; Gaina, G.; Ioghen, O.; Ceafalan, L.C.; Hinescu, M.E. Macrophages and Stem Cells-Two to Tango for Tissue Repair? Biomolecules 2021, 11, 697.
- Lin, W.; Xu, L.; Lin, S.; Shi, L.; Wang, B.; Pan, Q.; Lee, W.Y.W.; Li, G. Characterisation of multipotent stem cells from human peripheral blood using an improved protocol. J. Orthop. Transl. 2019, 19, 18–28.
- Cesselli, D.; Beltrami, A.P.; Rigo, S.; Bergamin, N.; D’Aurizio, F.; Verardo, R.; Piazza, S.; Klaric, E.; Fanin, R.; Toffoletto, B.; et al. Multipotent progenitor cells are present in human peripheral blood. Circ. Res. 2009, 104, 1225–1234.
- Jin, R.; Song, G.; Chai, J.; Gou, X.; Yuan, G.; Chen, Z. Effects of concentrated growth factor on proliferation, migration, and differentiation of human dental pulp stem cells in vitro. J. Tissue Eng. 2018, 9, 2041731418817505.
- Zhang, L.; Ai, H. Concentrated growth factor promotes proliferation, osteogenic differentiation, and angiogenic potential of rabbit periosteum-derived cells in vitro. J. Orthop. Surg. Res. 2019, 14, 146.
- Chen, J.; Jiang, H. A Comprehensive Review of Concentrated Growth Factors and Their Novel Applications in Facial Reconstructive and Regenerative Medicine. Aesthetic Plast. Surg. 2020, 44, 1047–1057.
- Calabriso, N.; Stanca, E.; Rochira, A.; Damiano, F.; Giannotti, L.; Di Chiara Stanca, B.; Massaro, M.; Scoditti, E.; Demitri, C.; Nitti, P.; et al. Angiogenic Properties of Concentrated Growth Factors (CGFs): The Role of Soluble Factors and Cellular Components. Pharmaceutics 2021, 13, 635.
- Everts, P.A.; Knape, J.T.; Weibrich, G.; Schönberger, J.P.; Hoffmann, J.; Overdevest, E.P.; Box, H.A.; van Zundert, A. Platelet-rich plasma and platelet gel: A review. J. Extra Corpor. Technol. 2006, 38, 174–187.
- Karaman, O.; Kumar, A.; Moeinzadeh, S.; He, X.; Cui, T.; Jabbari, E. Effect of surface modification of nanofibres with glutamic acid peptide on calcium phosphate nucleation and osteogenic differentiation of marrow stromal cells. J. Tissue Eng. Regen. Med. 2013, 10, E132–E146.
- Onak, G.; Şen, M.; Horzum, N.; Ercan, U.K.; Yaralı, Z.B.; Garipcan, B.; Karaman, O. Aspartic and Glutamic Acid Templated Peptides Conjugation on Plasma Modified Nanofibers for Osteogenic Differentiation of Human Mesenchymal Stem Cells: A Comparative Study. Sci. Rep. 2018, 8, 17620.
- Prideaux, M.; Kitase, Y.; Kimble, M.; O’Connell, T.M.; Bonewald, L.F. Taurine, an osteocyte metabolite, protects against oxidative stress-induced cell death and decreases inhibitors of the Wnt/β-catenin signaling pathway. Bone 2020, 137, 115374.
- Zhou, C.; Zhang, X.; Xu, L.; Wu, T.; Cui, L.; Xu, D. Taurine promotes human mesenchymal stem cells to differentiate into osteoblast through the ERK pathway. Amino Acids 2014, 46, 1673–1680.
- Bonazza, V.; Hajistilly, C.; Patel, D.; Patel, J.; Woo, R.; Cocchi, M.A.; Buffoli, B.; Lancini, D.; Gheno, E.; Rezzani, R.; et al. Growth Factors Release from Concentrated Growth Factors: Effect of β-Tricalcium Phosphate Addition. J. Craniofac. Surg. 2018, 29, 2291–2295.
- Huang, L.; Zou, R.; He, J.; Ouyang, K.; Piao, Z. Comparing osteogenic effects between concentrated growth factors and the acellular dermal matrix. Braz. Oral. Res. 2018, 32, e29.
- Dohan Ehrenfest, D.M.; Pinto, N.R.; Pereda, A.; Jime’nez, P.; Corso, M.D.; Kang, B.S.; Nally, M.; Lanata, N.; Wang, H.L.; Quirynen, M. The impact of the centrifuge characteristics and centrifugation protocols on the cells, growth factors, and fibrin architecture of a leukocyte- and platelet-rich fibrin (L-PRF) clot and membrane. Platelets 2018, 29, 171–184.
- Qin, J.; Wang, L.; Zheng, L.; Zhou, X.; Zhang, Y.; Yang, T.; Zhou, Y. Concentrated growth factor promotes Schwann cell migration partly through the integrin beta1-mediated activation of the focal adhesion kinase pathway. Int. J. Mol. Med. 2016, 37, 1363–1370.
- Honda, H.; Tamai, N.; Naka, N.; Yoshikawa, H.; Myoui, A. Bone tissue engineering with bone marrow-derived stromal cells integrated with concentrated growth factor in Rattus norvegicus calvaria defect model. J. Artif. Organs 2013, 16, 305–315.
- Wang, L.; Wan, M.; Li, Z.; Zhong, N.; Liang, D.; Ge, L. A comparative study of the effects of concentrated growth factors in two different forms on osteogenesis in vitro. Mol. Med. Rep. 2019, 20, 1039–1048.
- Isobe, K.; Watanebe, T.; Kawabata, H.; Kitamura, Y.; Kawase, T. Mechanical and degradation properties of advanced platelet-rich fibrin (APRF), concentrated growth factors (CGF), and platelet-poor plasma-derived fibrin (PPTF). Int. J. Implant Dent. 2017, 3, 17.
- Yu, M.; Wang, X.; Liu, Y.; Qiao, J. Cytokine release kinetics of concentrated growth factors in different scaffolds. Clin. Oral. Investig. 2018, 23, 1663–1671.
- Lei, L.; Yu, Y.; Han, J.; Shi, D.; Sun, W.; Zhang, D.; Chen, L. Quantification of growth factors in advanced platelet-rich fibrin and concentrated growth factors and their clinical efficacy as adjunctive to the GTR procedure in periodontal intrabony defects. J. Periodontol. 2020, 91, 462–472.
- Ferrara, N.; Gerber, H.-P. The Role of Vascular Endothelial Growth Factor in Angiogenesis. Acta Haematol. 2001, 106, 148–156.
- Ferrara, N.; Gerber, H.P.; LeCouter, J. The biology of VEGF and its receptors. Nat. Med. 2003, 9, 669–676.
- Lazarous, D.F.; Shou, M.; Scheinowitz, M.; Hodge, E.; Thirumurti, V.; Kitsiou, A.N.; Stiber, J.A.; Lobo, A.D.; Hunsberger, S.; Guetta, E.; et al. Comparative Effects of Basic Fibroblast Growth Factor and Vascular Endothelial Growth Factor on Coronary Collateral Development and the Arterial Response to Injury. Circulation 1996, 94, 1074–1082.
- Niu, G.; Chen, X. Vascular endothelial growth factor as an anti-angiogenic target for cancer therapy. Curr. Drug Targets 2010, 11, 1000–1017.
- Lee, K.; Silva, E.A.; Mooney, D.J. Growth factor delivery-based tissue engineering: General approaches and a review of recent developments. J. R. Soc. Interface 2011, 8, 153–170.
- Massagué, J.; Xi, Q. TGF-β control of stem cell differentiation genes. FEBS Lett. 2012, 586, 1953–1958.
- Li, M.O.; Flavell, R.A. TGF-β: A Master of All T Cell Trades. Cell 2008, 134, 392–404.
- Xue, T.; Wei, L.; Qiao, L.; Qiu, J.; Zha, D. Does bone morphogenetic proteins play an important role in chronic rhinosinusitis? Med. Hypotheses 2009, 72, 228.
- Kalén, A.; Wahlström, O.; Linder, C.H.; Magnusson, P. The content of bone morphogenetic proteins in platelets varies greatly between different platelet donors. Biochem. Biophys. Res. Commun. 2008, 375, 261–264.
- Zhang, Z.; Lai, Q.; Li, Y.; Xu, C.; Tang, X.; Ci, J.; Sun, S.; Xu, B.; Li, Y. Acidic pH environment induces autophagy in osteoblasts. Sci. Rep. 2017, 7, 46161.
- Caley, M.P.; Martins, V.L.; O’Toole, E.A. Metalloproteinases and Wound Healing. Adv. Wound Care (New Rochelle) 2015, 4, 225–234.
- Di Liddo, R.; Bertalot, T.; Borean, A.; Pirola, I.; Argentoni, A.; Schrenk, S.; Cenzi, C.; Capelli, S.; Conconi, M.T.; Parnigotto, P.P. Leucocyte and Platelet-rich Fibrin: A carrier of autologous multipotent cells for regenerative medicine. J. Cell Mol. Med. 2018, 22, 1840–1854.
- Kuwana, M.; Okazaki, Y.; Kodama, H.; Izumi, K.; Yasuoka, H.; Ogawa, Y.; Kawakami, Y.; Ikeda, Y. Human circulating CD14+ monocytes as a source of progenitors that exhibit mesenchymal cell differentiation. J. Leukoc. Biol. 2003, 74, 833–845.
- Kapellos, T.S.; Bonaguro, L.; Gemünd, I.; Reusch, N.; Saglam, A.; Hinkley, E.R.; Schultze, J.L. Human Monocyte Subsets and Phenotypes in Major Chronic Inflammatory Diseases. Front. Immunol. 2019, 10, 2035.
- Graf, T.; Stadtfeld, M. Heterogeneity of embryonic and adult stem cells. Cell Stem Cell 2008, 3, 480–483.
- Frucht, D.M.; Aringer, M.; Galon, J.; Danning, C.; Brown, M.; Fan, S.; Centola, M.; Wu, C.Y.; Yamada, N.; El Gabalawy, H.; et al. Stat4 is expressed in activated peripheral blood monocytes, dendritic cells, and macrophages at sites of Th1-mediated inflammation. J. Immunol. 2000, 164, 4659–4664.
- Amarasekara, D.S.; Yun, H.; Kim, S.; Lee, N.; Kim, H.; Rho, J. Regulation of Osteoclast Differentiation by Cytokine Networks. Immune Netw. 2018, 18, e8.
- Parra-Izquierdo, I.; Castaños-Mollor, I.; López, J.; Gómez, C.; San Román, J.A.; Sánchez Crespo, M.; García-Rodríguez, C. Calcification Induced by Type I Interferon in Human Aortic Valve Interstitial Cells Is Larger in Males and Blunted by a Janus Kinase Inhibitor. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 2148–2159.
More
