Robotic surgery has gained much attention in liver resection for its potential to increase surgical dexterity in a minimally invasive scenario. In liver surgery, robotic systems help surgeons to localize tumors and improve surgical results with well-defined preoperative planning or increased intraoperative detection. Furthermore, they can balance the absence of tactile feedback and help recognize intrahepatic biliary or vascular structures during parenchymal transection. In addition, the robotic system presents the advantage of creating a hybrid interface in which pre- and intra-operative imaging tools could be exploited alone or together in order to guide surgical resection.
- robotic
- liver surgery
- augmented reality
1. Introduction
2. Augmented Reality
Implementing technologies of robotic hepatic surgery through AR means overcoming the limitations widely described when performing liver resections through this approach. Despite the sense of depth achieved by the 3D visualization and the improved video resolution and magnification, the robotic system presents all the drawbacks of a minimally invasive view compared to an open hepatectomy. The absence of the tactile feedback and the unavailability of an ultrasonic dissector are undoubtedly the main issues found in this context. The importance of a tactile sensation is twofold in this kind of surgery. Firstly, from an oncological point of view, it helps locate the tumor and thus guide parenchymal transection. AR is effective in planning preoperatively the strategy through 3D rendering and, intraoperatively, targeting the lesion and resection margins (Figure 1) [17,18,19,20,21][17][18][19][20][21]. Buchs et al. [22], for example, overlaid the 3D preoperative images onto the endoscopic stream and added distance information in order to obtain a real-time guided transection. After projecting the tumor onto the liver surface and defining the margin width, a bar and a dartboard appeared on the screen to guide the tip of the instrument, with colors ranging from green to red to alert the operator if the distance to the tumor was respected or not. Ultrasound guidance or an intraoperative CT scan could be equally used, with the disadvantage of radiation, shifting the instruments constantly, or, occasionally, having difficulty localizing the nodules. In fact, AR has been described in open hepatectomies in the detection of vanishing metastasis, with the superimposition of lesions pre-existent to chemotherapy and undetectable at preoperative images or intraoperative US [23,24][23][24]. Secondly, the sense of touch aids the operator to orient him/herself in relation to some intra hepatic landmarks. Arteries, veins, and biliary structures, especially in the first-orders pedicles, present a thickened fibrotic sheath and the robotic “insensibility” can disorient surgeons during the dissection, with possible vascular injuries. Furthermore, lesions can be located in critical areas such as the hepatic confluence, and robotic dissection could thus be more complex [25]. AR-based intraoperative reconstructions and tracking systems may be used to map resection planes and show vascular structures during liver transection (Figure 1). Moreover, 3D planning can enable the identification of anatomical variants, as demonstrated in laparoscopic cholecystectomy [26]. These reconstructions could also represent a solution to the absence of some familiar devices in robotic liver surgery.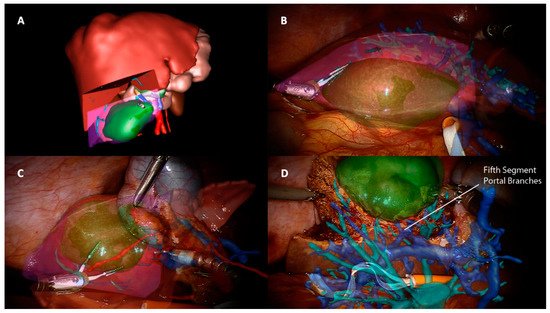
3. Image-Guided Robotic Liver Surgery
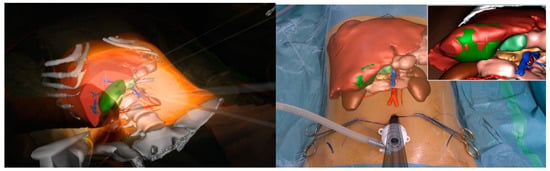
Other imaging strategies are more frequently used by surgeons during robotic liver resections in order to improve lesion detection and evaluate intraparenchymal biliary and vascular structures. Before exploring these modalities, a special mention goes to TilePro (Intuitive Surgical Inc., Sunnyvale, CA, USA). This is a multi-input display software integrated into the robotic platform, which shows more video sources simultaneously on the same screen (Figure 2). By simply connecting an external source to the Da Vinci console, surgeons and other operating room assistants can easily switch from the operating field to other input such as Intraoperative Ultrasound (IOUS) or preoperative cross-sectional imaging and 3D reconstructions.
3.1. Preoperative Imaging and 3D Rendering
3.2. Intra-Operative Robotic Ultrasound Application
IOUS is nowadays an essential tool for hepatobiliary surgeons. Several series emphasize its utility in tumor identification, the recognition of its spatial relationship with intrahepatic structures, and, therefore, in guiding parenchymal resection [38][30]. Furthermore, IOUS is demonstrated to be able to modify preoperative strategy in up to a quarter of cases because of different vascular relationships or new nodules found during surgical exploration, despite the performance of a liver-specific MRI [39,40,41][31][32][33]. Recently, a specific transducer was introduced to the robotic ecosystem with consequent better manageability, higher precision, and the creation of a multi-input environment. Surgeons can now easily control the probe, grabbing its dorsal fin with a forceps, and a highly flexible cable and a small transducer surface make the access to the posterior segments or to the inferior surface easier compared to the rigid laparoscopic counterpart [46,47][34][35]. Moreover, thanks to software such as TilePro, the operator can shift from the 3D camera view to the ultrasound directly from the console, or even create a split-view with both intraoperative and ultrasound images (Figure 43).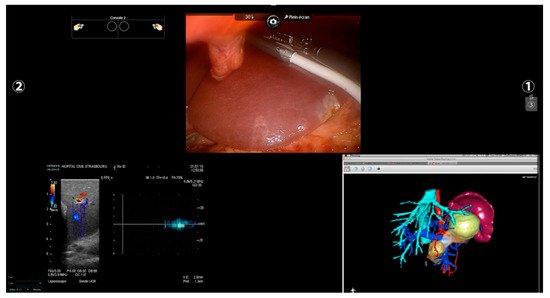
3.3. Indocyanine Green Fluorescence
Another aspect to consider in ICG-guided resections is tumor clearance. Minimally invasive approaches—and robotic, in particular—lack a tactile feedback, and achieving a parenchymal free margin or performing an anatomical resection could be challenging. Furthermore, an IOUS exclusive evaluation could be insufficient because it is a user-dependent procedure and presents a heterogeneous detection rate according to tumor size and location and parenchymal stiffness [62,63,64][48][49][50]. In this context, fluorescence is a precious tool in robotic surgery, with some authors reporting an enlargement of the resection area after ICG application, both in benign ad malignant lesions, in order to achieve a R0 resection [57[43][51][52][53],65,66,67], and a significantly higher rate of margin-free specimens when comparing robotic hepatectomies with and without ICG [67][53]. As in open surgery, even in robotic surgery, some series described the detection of newer superficial lesions that the dye injection missed before [57][43]. This high sensitivity found is, however, limited to the liver surface because of the low penetration of the dye under 8 mm of depth, thus requiring the use of other imaging tools such as IOUS. Although no long-term results have been published, these findings have a significant impact in terms of oncological outcomes. ICG is a promising instrument of intraoperative navigation surgery, allowing rapid and easy identification of the resection plane without the inconveniences mentioned for other image-guided techniques. It can be used in combination with IOUS or AR as an additional aid rather than as a replacement [61] [47] and with its features, it seems to fill some gaps found in robotic surgery, making tailored and oncological surgery less challenging.
4. Future Prospective
liver experience, mainly due to some technical limitations and to a relatively newborn and still debated approach [15]. AR, for example, is a time-consuming procedure, not only for the intraoperative installation, but also for preoperative planning and liver rendering [68][54]. In the context of an atypical or less demanding hepatic resection, which represent the first steps of a necessary learning curve, this time could appear exaggerated. Furthermore, AR in hepatic surgery has showed a delayed distribution compared to other surgical fields as neurosurgery, otolaryngology, orthopedics, and maxillofacial surgery [69,70,71][55][56][57]. This difference comes from anatomical obstacles, such as working with a deformable soft organ that is constantly moving during operation because of respiratory cycles as well as pneumoperitoneum creation [72][58]. Although some strategies have been described in this context [23[23][59][60],73,74], these features make the development of AR more complex, and new software are needed for shortening modeling creation and improving the accuracy of manual, semiautomatic, and automatic images overlapping.
All the imaging techniques described must be seen, however, as a part of a puzzle rather than an independent solution towards a guided surgery; an example comes from registration accuracy in AR. IOUS and ICG have been proposed to improve overlapping quality through fluorescent markers and 3D ultrasounds used for intraoperative landmarks [75,76][61][62]. In this scenario, the robotic platform fits perfectly by creating a unique merged environment with the possibility of using and visualizing preoperative reconstruction and intraoperative images simultaneously within the operative field (Figure 43).
Another potential benefit of image-guided technology is minimally invasive training.
In laparoscopy, telementoring based on AR seems to speed up simple skills acquisition such as suturing [77][63] or even reduce the learning curve in more complex procedures such as cholecystectomy [78][64]. Similar applications in robotic training are lacking, with only a few experiences described [79][65]. Hepato-biliary surgery lacks standards of training and learning curves in robotic procedures [80][66], but recently, an expert panel of HPB surgeons agreed that a correct training path in hepatobiliary procedures needs different steps, starting from basic robotic skills before performing a liver resection [81][67]. In this context, AR could be a useful tool to support less-experienced surgeons performing simple procedures and lower their learning curve.
5. Conclusions
References
- Ciria, R.; Cherqui, D.; Geller, D.A.; Briceno, J.; Wakabayashi, G. Comparative Short-term Benefits of Laparoscopic Liver Resection. Ann. Surg. 2016, 263, 761–777.
- Wakabayashi, G.; Cherqui, D.; Geller, D.A.; Buell, J.F.; Kaneko, H.; Han, H.S.; Asbun, H.; O’rourke, N.; Tanabe, M.; Koffron, A.J.; et al. Recommendations for laparoscopic liver resection: A report from the second international consensus conference held in Morioka. Ann. Surg. 2015, 261, 619–629.
- Kasai, M.; Cipriani, F.; Gayet, B.; Aldrighetti, L.; Ratti, F.; Sarmiento, J.M.; Scatton, O.; Kim, K.-H.; Dagher, I.; Topal, B.; et al. Laparoscopic versus open major hepatectomy: A systematic review and meta-analysis of individual patient data. Surgery 2018, 163, 985–995.
- Machairas, N.; Kostakis, I.D.; Schizas, D.; Kykalos, S.; Nikiteas, N.; Sotiropoulos, G.C. Meta-analysis of laparoscopic versus open liver resection for intrahepatic cholangiocarcinoma. Updates Surg. 2020, 73, 59–68.
- Guerrini, G.P.; Esposito, G.; Tarantino, G.; Serra, V.; Olivieri, T.; Catellani, B.; Assirati, G.; Guidetti, C.; Ballarin, R.; Magistri, P.; et al. Laparoscopic versus open liver resection for intrahepatic cholangiocarcinoma: The first meta-analysis. Langenbeck’s Arch. Surg. 2020, 405, 265–275.
- Sotiropoulos, G.C.; Prodromidou, A.; Kostakis, I.D.; Machairas, N. Meta-analysis of laparoscopic vs open liver resection for hepatocellular carcinoma. Updates Surg. 2017, 69, 291–311.
- Syn, N.L.; Kabir, T.; Koh, Y.X.; Tan, H.L.; Wang, L.Z.; Chin, B.Z.; Wee, I.; Teo, J.Y.; Tai, B.C.; Goh, B.K.P. Survival Advantage of Laparoscopic Versus Open Resection For Colorectal Liver Metastases. Ann. Surg. 2019, 272, 253–265.
- Schmelzle, M.; Krenzien, F.; Schöning, W.; Pratschke, J. Laparoscopic liver resection: Indications, limitations, and economic aspects. Langenbeck’s Arch. Surg. 2020, 405, 725–735.
- Tsung, A.; Geller, D.A.; Sukato, D.C.; Sabbaghian, S.; Tohme, S.; Steel, J.; Marsh, W.; Reddy, S.K.; Bartlett, D.L. Robotic Versus Laparoscopic Hepatectomy. Ann. Surg. 2014, 259, 549–555.
- Gavriilidis, P.; Roberts, K.J.; Aldrighetti, L.; Sutcliffe, R.P. A comparison between robotic, laparoscopic and open hepatectomy: A systematic review and network meta-analysis. Eur. J. Surg. Oncol. 2020, 46, 1214–1224.
- Zhao, Z.; Yin, Z.; Li, M.; Jiang, N.; Liu, R. State of the art in robotic liver surgery: A meta-analysis. Updates Surg. 2020, 73, 977–987.
- Zhang, L.; Yuan, Q.; Xu, Y.; Wang, W. Comparative clinical outcomes of robot-assisted liver resection versus laparoscopic liver resection: A meta-analysis. PLoS ONE 2020, 15, e0240593.
- Machairas, N.; Papaconstantinou, D.; Tsilimigras, D.I.; Moris, D.; Prodromidou, A.; Paspala, A.; Spartalis, E.; Kostakis, I.D. Comparison between robotic and open liver resection: A systematic review and meta-analysis of short-term outcomes. Updates Surg. 2019, 71, 39–48.
- Tsilimigras, D.I.; Moris, D.; Vagios, S.; Merath, K.; Pawlik, T.M. Safety and oncologic outcomes of robotic liver resections: A systematic review. J. Surg. Oncol. 2018, 117, 1517–1530.
- Liu, R.; Wakabayashi, G.; Kim, H.-J.; Choi, G.-H.; Yiengpruksawan, A.; Fong, Y.; He, J.; Boggi, U.; Troisi, R.I.; Efanov, M.; et al. International consensus statement on robotic hepatectomy surgery in 2018. World J. Gastroenterol. 2019, 25, 1432–1444.
- Giménez, M.; Gallix, B.; Costamagna, G.; Vauthey, J.-N.; Moche, M.; Wakabayashi, G.; Bale, R.; Swanström, L.; Futterer, J.; Geller, D.; et al. Definitions of Computer-Assisted Surgery and Intervention, Image-Guided Surgery and Intervention, Hybrid Operating Room, and Guidance Systems. Ann. Surg. Open 2020, 1, e21.
- Okuda, Y.; Taura, K.; Seo, S.; Yasuchika, K.; Nitta, T.; Ogawa, K.; Hatano, E.; Uemoto, S. Usefulness of operative planning based on 3-dimensional CT cholangiography for biliary malignancies. Surgery 2015, 158, 1261–1271.
- Fang, C.-H.; Tao, H.-S.; Yang, J.; Fang, Z.-S.; Cai, W.; Liu, J.; Fan, Y.-F. Impact of Three-Dimensional Reconstruction Technique in the Operation Planning of Centrally Located Hepatocellular Carcinoma. J. Am. Coll. Surg. 2014, 220, 28–37.
- Hallet, J.; Soler, L.; Diana, M.; Mutter, D.; Baumert, T.F.; Habersetzer, F.; Marescaux, J.; Pessaux, P. Trans-Thoracic Minimally Invasive Liver Resection Guided by Augmented Reality. J. Am. Coll. Surg. 2015, 220, e55–e60.
- Phutane, P.; Buc, E.; Poirot, K.; Ozgur, E.; Pezet, D.; Bartoli, A.; Le Roy, B. Preliminary trial of augmented reality performed on a laparoscopic left hepatectomy. Surg. Endosc. 2017, 32, 514–515.
- Mise, Y.; Hasegawa, K.; Satou, S.; Shindoh, J.; Miki, K.; Akamatsu, N.; Arita, J.; Kaneko, J.; Sakamoto, Y.; Kokudo, N. How Has Virtual Hepatectomy Changed the Practice of Liver Surgery? Ann. Surg. 2018, 268, 127–133.
- Buchs, N.C.; Volonte, F.; Pugin, F.; Toso, C.; Fusaglia, M.; Gavaghan, K.; Majno, P.E.; Peterhans, M.; Weber, S.; Morel, P. Augmented environments for the targeting of hepatic lesions during image-guided robotic liver surgery. J. Surg. Res. 2013, 184, 825–831.
- Ntourakis, D.; Memeo, R.; Soler, L.; Marescaux, J.; Mutter, D.; Pessaux, P. Augmented Reality Guidance for the Resection of Missing Colorectal Liver Metastases: An Initial Experience. World J. Surg. 2015, 40, 419–426.
- Kingham, T.P.; Pak, L.M.; Simpson, A.L.; Leung, U.; Doussot, A.; D’Angelica, M.I.; DeMatteo, R.P.; Allen, P.J.; Jarnagin, W.R. 3D image guidance assisted identification of colorectal cancer liver metastases not seen on intraoperative ultrasound: Results from a prospective trial. HPB 2017, 20, 260–267.
- Banz, V.M.; Müller, P.C.; Tinguely, P.; Inderbitzin, D.; Ribes, D.; Peterhans, M.; Candinas, D.; Weber, S. Intraoperative image-guided navigation system: Development and applicability in 65 patients undergoing liver surgery. Langenbeck’s Arch. Surg. 2016, 401, 495–502.
- Diana, M.; Soler, L.; Agnus, V.; D’Urso, A.; Vix, M.; Dallemagne, B.; Faucher, V.; Roy, C.; Mutter, D.; Marescaux, J.; et al. Prospective Evaluation of Precision Multimodal Gallbladder Surgery Navigation. Ann. Surg. 2017, 266, 890–897.
- Pessaux, P.; Diana, M.; Soler, L.; Piardi, T.; Mutter, D.; Marescaux, J. Towards cybernetic surgery: Robotic and augmented reality-assisted liver segmentectomy. Langenbeck’s Arch. Surg. 2014, 400, 381–385.
- Marescaux, J.; Clément, J.-M.; Tassetti, V.; Koehl, C.; Cotin, S.; Russier, Y.; Mutter, D.; Delingette, H.; Ayache, N. Virtual Reality Applied to Hepatic Surgery Simulation: The Next Revolution. Ann. Surg. 1998, 228, 627–634.
- Palomar, R.; Cheikh, F.A.; Edwin, B.; Fretland, Å.; Beghdadi, A.; Elle, O.J. A novel method for planning liver resections using deformable Bézier surfaces and distance maps. Comput. Methods Programs Biomed. 2017, 144, 135–145.
- Araki, K.; Conrad, C.; Ogiso, S.; Kuwano, H.; Gayet, B. Intraoperative Ultrasonography of Laparoscopic Hepatectomy: Key Technique for Safe Liver Transection. J. Am. Coll. Surg. 2013, 218, e37–e41.
- Ferrero, A.; Langella, S.; Giuliante, F.; Viganò, L.; Vellone, M.; Zimmitti, G.; Ardito, F.; Nuzzo, G.; Capussotti, L. Intraoperative Liver Ultrasound Still Affects Surgical Strategy for Patients with Colorectal Metastases in the Modern Era. World J. Surg. 2013, 37, 2655–2663.
- Langella, S.; Ardito, F.; Russolillo, N.; Panettieri, E.; Perotti, S.; Mele, C.; Giuliante, F.; Ferrero, A. Intraoperative Ultrasound Staging for Colorectal Liver Metastases in the Era of Liver-Specific Magnetic Resonance Imaging: Is It Still Worthwhile? J. Oncol. 2019, 2019, 1369274.
- D’Hondt, M.; Vandenbroucke-Menu, F.; Préville-Ratelle, S.; Turcotte, S.; Chagnon, M.; Plasse, M.; Létourneau, R.; Dagenais, M.; Roy, A.; Lapointe, R. Is intra-operative ultrasound still useful for the detection of a hepatic tumour in the era of modern pre-operative imaging? HPB 2011, 13, 665–669.
- Cho, J.Y. Outcomes of Laparoscopic Liver Resection for Lesions Located in the Right Side of the Liver. Arch. Surg. 2009, 144, 25–29.
- Casciola, L.; Patriti, A.; Ceccarelli, G.; Bartoli, A.; Ceribelli, C.; Spaziani, A. Robot-assisted parenchymal-sparing liver surgery including lesions located in the posterosuperior segments. Surg. Endosc. 2011, 25, 3815–3824.
- Majlesara, A.; Golriz, M.; Hafezi, M.; Saffari, A.; Stenau, E.; Maier-Hein, L.; Müller-Stich, B.P.; Mehrabi, A. Indocyanine green fluorescence imaging in hepatobiliary surgery. Photodiagnosis Photodyn. Ther. 2017, 17, 208–215.
- Reinhart, M.B.; Huntington, C.R.; Blair, L.J.; Heniford, B.T.; Augenstein, V.A. Indocyanine Green. Surg. Innov. 2015, 23, 166–175.
- Daskalaki, D.; Aguilera, F.; Patton, K.; Giulianotti, P.C. Fluorescence in robotic surgery. J. Surg. Oncol. 2015, 112, 250–256.
- Handgraaf, H.; Boogerd, L.; Höppener, D.; Peloso, A.; Mulder, B.S.; Hoogstins, C.; Hartgrink, H.; van de Velde, C.; Mieog, J.; Swijnenburg, R.; et al. Long-term follow-up after near-infrared fluorescence-guided resection of colorectal liver metastases: A retrospective multicenter analysis. Eur. J. Surg. Oncol. 2017, 43, 1463–1471.
- Marino, M.V.; Podda, M.; Fernandez, C.C.; Ruiz, M.G.; Fleitas, M.G. The application of indocyanine green-fluorescence imaging during robotic-assisted liver resection for malignant tumors: A single-arm feasibility cohort study. HPB 2020, 22, 422–431.
- Van Der Vorst, J.R.; Schaafsma, B.E.; Hutteman, M.; Msc, F.P.R.V.; Liefers, G.-J.; Hartgrink, H.H.; Smit, V.T.H.B.M.; Löwik, C.W.G.M.; Van De Velde, C.J.H.; Frangioni, J.V.; et al. Near-infrared fluorescence-guided resection of colorectal liver metastases. Cancer 2013, 119, 3411–3418.
- Hoekstra, L.T.; de Graaf, W.; Nibourg, G.A.A.; Heger, M.; Bennink, R.J.; Stieger, B.; van Gulik, T.M. Physiological and Biochemical Basis of Clinical Liver Function Tests. Ann. Surg. 2013, 257, 27–36.
- Marino, M.V.; Podda, M.; Fernandez, C.C.; Ruiz, M.G.; Fleitas, M.G. The application of indocyanine green-fluorescence imaging during robotic-assisted liver resection for malignant tumors: A single-arm feasibility cohort study. HPB 2020, 22, 422–431.
- Van Der Vorst, J.R.; Schaafsma, B.E.; Hutteman, M.; Msc, F.P.R.V.; Liefers, G.-J.; Hartgrink, H.H.; Smit, V.T.H.B.M.; Löwik, C.W.G.M.; Van De Velde, C.J.H.; Frangioni, J.V.; et al. Near-infrared fluorescence-guided resection of colorectal liver metastases. Cancer 2013, 119, 3411–3418.
- Hoekstra, L.T.; de Graaf, W.; Nibourg, G.A.A.; Heger, M.; Bennink, R.J.; Stieger, B.; van Gulik, T.M. Physiological and Biochemical Basis of Clinical Liver Function Tests. Ann. Surg. 2013, 257, 27–36.
- Cheung, T.T.; Ma, K.W.; She, W.H.; Dai, W.C.; Tsang, S.H.Y.; Chan, A.C.Y.; Chok, K.S.H.; Lo, C.M. Pure laparoscopic hepatectomy with augmented reality-assisted indocyanine green fluorescence versus open hepatectomy for hepatocellular carcinoma with liver cirrhosis: A propensity analysis at a single center. Asian J. Endosc. Surg. 2018, 11, 104–111.
- Chiow, A.K.H.; Rho, S.Y.; Wee, I.J.; Lee, L.S.; Choi, G.H. Robotic ICG guided anatomical liver resection in a multi-centre cohort: An evolution from “positive staining” into “negative staining” method. HPB 2020, 23, 475–482.
- Ishizawa, T.; Fukushima, N.; Shibahara, J.; Masuda, K.; Tamura, S.; Aoki, T.; Hasegawa, K.; Beck, Y.; Fukayama, M.; Kokudo, N. Real-time identification of liver cancers by using indocyanine green fluorescent imaging. Cancer 2009, 115, 2491–2504.
- Boogerd, L.S.F.; Handgraaf, H.; Lam, H.-D.; Huurman, V.A.L.; Sarasqueta, A.F.; Frangioni, J.V.; Van De Velde, C.J.H.; Braat, A.E.; Vahrmeijer, A.L. Laparoscopic detection and resection of occult liver tumors of multiple cancer types using real-time near-infrared fluorescence guidance. Surg. Endosc. 2016, 31, 952–961.
- Peloso, A.; Franchi, E.; Canepa, M.C.; Barbieri, L.; Briani, L.; Ferrario, J.; Bianco, C.; Quaretti, P.; Brugnatelli, S.; Dionigi, P.; et al. Combined use of intraoperative ultrasound and indocyanine green fluorescence imaging to detect liver metastases from colorectal cancer. HPB 2013, 15, 928–934.
- Li, C.-G.; Zhou, Z.-P.; Tan, X.-L.; Wang, Z.-Z.; Liu, Q.; Zhao, Z.-M. Robotic resection of liver focal nodal hyperplasia guided by indocyanine green fluorescence imaging: A preliminary analysis of 23 cases. World J. Gastrointest. Oncol. 2020, 12, 1407–1415.
- Mehdorn, A.-S.; Beckmann, J.; Braun, F.; Becker, T.; Egberts, J.-H. Usability of Indocyanine Green in Robot-Assisted Hepatic Surgery. J. Clin. Med. 2021, 10, 456.
- Marino, M.V.; Di Saverio, S.; Podda, M.; Ruiz, M.G.; Fleitas, M.G. The Application of Indocyanine Green Fluorescence Imaging During Robotic Liver Resection: A Case-Matched Study. World J. Surg. 2019, 43, 2595–2606.
- Okamoto, T.; Onda, S.; Yanaga, K.; Suzuki, N.; Hattori, A. Clinical application of navigation surgery using augmented reality in the abdominal field. Surg. Today 2014, 45, 397–406.
- Meola, A.; Cutolo, F.; Carbone, M.; Cagnazzo, F.; Ferrari, M.; Ferrari, V. Augmented reality in neurosurgery: A systematic review. Neurosurg. Rev. 2016, 40, 537–548.
- Jud, L.; Fotouhi, J.; Andronic, O.; Aichmair, A.; Osgood, G.; Navab, N.; Farshad, M. Applicability of augmented reality in orthopedic surgery—A systematic review. BMC Musculoskelet. Disord. 2020, 21, 103.
- Ayoub, A.; Pulijala, Y. The application of virtual reality and augmented reality in Oral & Maxillofacial Surgery. BMC Oral Health 2019, 19, 238.
- Teatini, A.; de Frutos, J.P.; Eigl, B.; Pelanis, E.; Aghayan, D.L.; Lai, M.; Kumar, R.P.; Palomar, R.; Edwin, B.; Elle, O.J. Influence of sampling accuracy on augmented reality for laparoscopic image-guided surgery. Minim. Invasive Ther. Allied Technol. 2020, 30, 229–238.
- Kenngott, H.G.; Wagner, M.; Gondan, M.; Nickel, F.; Nolden, M.; Fetzer, A.; Weitz, J.; Fischer, L.; Speidel, S.; Meinzer, H.-P.; et al. Real-time image guidance in laparoscopic liver surgery: First clinical experience with a guidance system based on intraoperative CT imaging. Surg. Endosc. 2013, 28, 933–940.
- Luo, H.; Yin, D.; Zhang, S.; Xiao, D.; He, B.; Meng, F.; Zhang, Y.; Cai, W.; He, S.; Zhang, W.; et al. Augmented reality navigation for liver resection with a stereoscopic laparoscope. Comput. Methods Programs Biomed. 2019, 187, 105099.
- Nam, W.H.; Kang, D.-G.; Lee, D.; Lee, J.Y.; Ra, J.B. Automatic registration between 3D intra-operative ultrasound and pre-operative CT images of the liver based on robust edge matching. Phys. Med. Biol. 2011, 57, 69–91.
- Kong, S.-H.; Haouchine, N.; Soares, R.; Klymchenko, A.S.; Andreiuk, B.; Marques, B.; Shabat, G.; Piechaud, T.; Diana, M.; Cotin, S.; et al. Robust augmented reality registration method for localization of solid organs’ tumors using CT-derived virtual biomechanical model and fluorescent fiducials. Surg. Endosc. 2016, 31, 2863–2871.
- Vera, A.M.; Russo, M.; Mohsin, A.; Tsuda, S. Augmented reality telementoring (ART) platform: A randomized controlled trial to assess the efficacy of a new surgical education technology. Surg. Endosc. 2014, 28, 3467–3472.
- Kowalewski, K.-F.; Garrow, C.; Proctor, T.; Preukschas, A.A.; Friedrich, M.; Müller, P.C.; Kenngott, H.G.; Fischer, L.; Müller-Stich, B.P.; Nickel, F. LapTrain: Multi-modality training curriculum for laparoscopic cholecystectomy—results of a randomized controlled trial. Surg. Endosc. 2018, 32, 3830–3838.
- Lee, J.H.; Tanaka, E.; Woo, Y.; Ali, G.; Son, T.; Kim, H.; Hyung, W.J. Advanced real-time multi-display educational system (ARMES): An innovative real-time audiovisual mentoring tool for complex robotic surgery. J. Surg. Oncol. 2017, 116, 894–897.
- Lai, E.C.; Tang, C.N. Training robotic hepatectomy: The Hong Kong experience and perspective. Hepatobiliary Surg. Nutr. 2017, 6, 222–229.
- Fong, Y.; Buell, J.F.; Collins, J.; Martinie, J.; Bruns, C.; Tsung, A.; Clavien, P.-A.; Nachmany, I.; Edwin, B.; Pratschke, J.; et al. Applying the Delphi process for development of a hepatopancreaticobiliary robotic surgery training curriculum. Surg. Endosc. 2020, 34, 4233–4244.
