HIV-1 cell-to-cell transmission is key for an effective viral replication that evades immunity. This highly infectious mechanism is orchestrated by different cellular targets that utilize a wide variety of processes to efficiently transfer HIV-1 particles. Dendritic cells (DCs) are the most potent antigen presenting cells that initiate antiviral immune responses, but are also the cells with highest capacity to transfer HIV-1 through cell-to-cell contacts.
- dendritic cells (DCs)
- HIV-1
1. Introduction
Several viruses have the ability to hijack pre-existing mechanisms of cellular communication to facilitate direct cell-to-cell viral spread [1][2][3], and the human immunodeficiency virus type 1 (HIV-1) is not an exception [1][4]. Before the definition of the precise mechanisms of cell-to-cell viral transmission, early studies highlighted the increased efficiency of HIV-1 spread by cellular contacts as compared to the diffusion-limited movement of free viral particles, suggesting that cell-to-cell dissemination might be up to 1000 times more efficient [5]. However, the first detailed description of a stable cellular junction between infected and non-infected cells to facilitate viral spread, known as virological synapse (VS), was reported for the human T cell leukaemia virus type 1 (HTLV-1), which is inefficient at infecting T cells and requires cellular contacts for effective spread [6]. Soon after this description, several studies showed co-clustering of HIV-1 proteins with their receptors CD4 and CXCR4, together with a massive viral transmission at the stable interface formed between HIV-1-infected and non-infected CD4+ T cells [7][8], thus expanding the concept of VS to HIV-1.
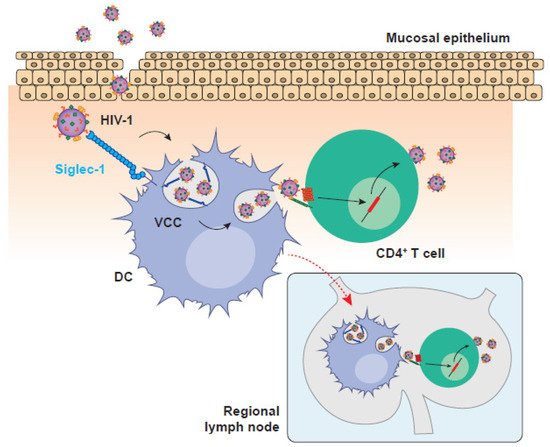
In addition to the VS, there is another type of synapse formed between antigen presenting cells (APCs) such as DCs and CD4+ T cells, which can even operate in the absence of productive infection of the donor APC. During antigen presentation, the formation ofcognate DC:T cell conjugates or ‘immunological synapses’ is necessary for the activation of T cells [9][10]. Once activated, T cells proliferate and differentiate into effector cells, which mediate adaptive immune responses aimed to eliminate invading viruses [11]. Intriguingly, upon HIV-1 infection, the intimate cell-to-cell contacts formed between DCs and CD4+ T cells can boost viral transmission via the formation of an ‘infectious synapse’ [12] that allows for systemic HIV-1 dissemination.
2. Breaking the Ice: DCs Orchestrate Immune Responses against HIV-1 and Other Viruses
3. When Immunity Is Put on Ice: DCs as Promoters of HIV-1 Cell-to-cell Transmission
References
- Dustin, M.L.; Chakraborty, A.K.; Shaw, A.S. Understanding the Structure and Function of the Immunological Synapse. Cold Spring Harb. Perspect. Biol. 2010, 2, a002311.
- Basu, R.; Huse, M. Mechanical Communication at the Immunological Synapse. Trends Cell Biol. 2017, 27, 241–254.
- Hildreth, A.D.; O’sullivan, T.E. Tissue-Resident Innate and Innate-like Lymphocyte Responses to Viral Infection. Viruses 2019, 11, 272.
- Piguet, V.; Steinman, R.M. The Interaction of HIV with Dendritic Cells: Outcomes and Pathways. Trends Immunol. 2007, 28, 503–510.
- Steinman, R.M. The Dendritic Cell System and Its Role in Immunogenicity. Annu. Rev. Immunol. 1991, 9, 271–296.
- Banchereau, J.; Steinman, R.M. Dendritic Cells and the Control of Immunology. Nature 1998, 392, 245–252.
- Jolly, C.; Kashefi, K.; Hollinshead, M.; Sattentau, Q.J. HIV-1 Cell to Cell Transfer across an Env-Induced, Actin-Dependent Synapse. J. Exp. Med. 2004, 199, 283–293.
- Blanco, J.; Bosch, B.; Fernández-Figueras, M.T.; Barretina, J.; Clotet, B.; Esté, J.A. High Level of Coreceptor-Independent HIV Transfer Induced by Contacts between Primary CD4 T Cells. J. Biol. Chem. 2004, 279, 51305–51314.
- Paludan, C.; Schmid, D.; Landthaler, M.; Vockerodt, M.; Kube, D.; Tuschl, T.; Münz, C. Endogenous MHC Class II Processing of a Viral Nuclear Antigen after Autophagy. Science 2005, 307, 593–596.
- Wilson, N.S.; Behrens, G.M.N.; Lundie, R.J.; Smith, C.M.; Waithman, J.; Young, L.; Forehan, S.P.; Mount, A.; Steptoe, R.J.; Shortman, K.D.; et al. Systemic Activation of Dendritic Cells by Toll-like Receptor Ligands or Malaria Infection Impairs Cross-Presentation and Antiviral Immunity. Nat. Immunol. 2006, 7, 165–172.
- Steinman, R.M. The Dendritic Cell System and Its Role in Immunogenicity. Annu. Rev. Immunol. 1991, 9, 271–296.
- Banchereau, J.; Steinman, R.M. Dendritic Cells and the Control of Immunology. Nature 1998, 392, 245–252.
- Janeway, C.A.; Medzhitov, R. Innate Immune Recognition. Annu. Rev. Immunol. 2002, 20, 197–216.
- Cui, J.; Chen, Y.; Wang, H.Y.; Wang, R.F. Mechanisms and Pathways of Innate Immune Activation and Regulation in Health and Cancer. Hum. Vaccines Immunother. 2014, 10, 3270–3285.
- Janeway, C.A. Pillars Article: Approaching the Asymptote? Evolution and Revolution in Immunology. Cold Spring Harb Symp Quant Biol. 1989. 54: 1-13. J. Immunol. 2013, 191, 4475–4487.
- Medzhitov, R.; Preston-Hurlburt, P.; Janeway, C.A. A Human Homologue of the Drosophila Toll Protein Signals Activation of Adaptive Immunity. Nature 1997, 388, 394–397.
- Poltorak, A.; Smirnova, I.; He, X.; Liu, M.-Y.; Van Huffel, C.; Birdwell, D.; Alejos, E.; Silva, M.; Du, X.; Thompson, P.; et al. Genetic and Physical Mapping of TheLpsLocus: Identification of the Toll-4 Receptor as a Candidate Gene in the Critical Region. Blood Cells Mol. Dis. 1998, 24, 340–355.
- O’Neill, L.A.J.; Golenbock, D.; Bowie, A.G. The History of Toll-like Receptors—Redefining Innate Immunity. Nat. Rev. Immunol. 2013, 13, 453–460.
- Geijtenbeek, T.B.H.; Kwon, D.S.; Torensma, R.; van Vliet, S.J.; van Duijnhoven, G.C.F.; Middel, J.; Cornelissen, I.L.M.H.A.; Nottet, H.S.L.M.; KewalRamani, V.N.; Littman, D.R.; et al. DC-SIGN, a Dendritic Cell-Specific HIV-1-Binding Protein That Enhances Trans-Infection of T Cells. Cell 2000, 100, 587–597.
- Zhang, F.; Ren, S.; Zuo, Y. DC-SIGN, DC-SIGNR and LSECtin: C-Type Lectins for Infection. Int. Rev. Immunol. 2014, 33, 54–66.
- Powell, L.D.; Varki, A. I-Type Lectins. J. Biol. Chem. 1995, 270, 14243–14246.
- Angata, T.; Brinkman-Van der Linden, E.C.M. I-Type Lectins. Biochim. Biophys. Acta (BBA)—Gen. Subj. 2002, 1572, 294–316.
- Crocker, P.R.; Paulson, J.C.; Varki, A. Siglecs and Their Roles in the Immune System. Nat. Rev. Immunol. 2007, 7, 255–266.
- Collin, M.; Bigley, V. Human Dendritic Cell Subsets: An Update. Immunology 2018, 154, 3–20.
- Rhodes, J.W.; Tong, O.; Harman, A.N.; Turville, S.G. Human Dendritic Cell Subsets, Ontogeny, and Impact on HIV Infection. Front. Immunol. 2019, 10, 1008.
- Siegal, F.P.; Kadowaki, N.; Shodell, M.; Fitzgerald-Bocarsly, P.A.; Shah, K.; Ho, S.; Antonenko, S.; Liu, Y.-J. The Nature of the Principal Type 1 Interferon-Producing Cells in Human Blood. Science 1999, 284, 1835–1837.
- Worbs, T.; Hammerschmidt, S.I.; Förster, R. Dendritic Cell Migration in Health and Disease. Nat. Rev. Immunol. 2017, 17, 30–48.
- Hashimoto, M.; Im, S.J.; Araki, K.; Ahmed, R. Cytokine-Mediated Regulation of CD8 T-Cell Responses during Acute and Chronic Viral Infection. Cold Spring Harb. Perspect. Biol. 2019, 11, a028464.
- Mantegazza, A.R.; Magalhaes, J.G.; Amigorena, S.; Marks, M.S. Presentation of Phagocytosed Antigens by MHC Class I and II. Traffic 2013, 14, 135–152.
- Huang, Q.; Hu, J.; Tang, J.; Xu, L.; Ye, L. Molecular Basis of the Differrentiation and Function of Virus Specific Follicular Helper CD4+ T Cells. Front. Immunol. 2019, 10, 249.
- Joffre, O.P.; Segura, E.; Savina, A.; Amigorena, S. Cross-Presentation by Dendritic Cells. Nat. Rev. Immunol. 2012, 12, 557–569.
- Smed-Sörensen, A.; Chalouni, C.; Chatterjee, B.; Cohn, L.; Blattmann, P.; Nakamura, N.; Delamarre, L.; Mellman, I. Influenza a Virus Infection of Human Primary Dendritic Cells Impairs Their Ability to Cross-Present Antigen to CD8 T Cells. PLoS Pathog. 2012, 8, e1002572.
- Bender, A.; Bui, L.K.; Feldman, M.A.; Larsson, M.; Bhardwaj, N. Inactivated Influenza Virus, When Presented on Dendritic Cells, Elicits Human CD8+ Cytolytic T Cell Responses. J. Exp. Med. 1995, 182, 1663–1671.
- Larsson, M.; Fonteneau, J.-F.; Lirvall, M.; Haslett, P.; Lifson, J.D.; Bhardwaj, N. Activation of HIV-1 Specific CD4 and CD8 T Cells by Human Dendritic Cells: Roles for Cross-Presentation and Non-Infectious HIV-1 Virus. AIDS 2002, 16, 1319–1329.
- JACOBSON, S.; SEKALY, R.P.; BELLINI, W.J.; JOHNSON, C.L.; McFARLAND, H.F.; LONG, E.O. Recognition of Intracellular Measles Virus Antigens by HLA Class II Restricted Measles Virus-Specific Cytotoxic T Lymphocytes. Ann. N. Y. Acad. Sci. 1988, 540, 352–353.
- Miller, M.A.; Ganesan, A.P.V.; Luckashenak, N.; Mendonca, M.; Eisenlohr, L.C. Endogenous Antigen Processing Drives the Primary CD4+ T Cell Response to Influenza. Nat. Med. 2015, 21, 1216–1222.
- Coulon, P.-G.; Richetta, C.; Rouers, A.; Blanchet, F.P.; Urrutia, A.; Guerbois, M.; Piguet, V.; Theodorou, I.; Bet, A.; Schwartz, O.; et al. HIV-Infected Dendritic Cells Present Endogenous MHC Class II–Restricted Antigens to HIV-Specific CD4+ T Cells. J. Immunol. 2016, 197, 517–532.
- Piguet, V.; Steinman, R.M. The Interaction of HIV with Dendritic Cells: Outcomes and Pathways. Trends Immunol. 2007, 28, 503–510.
- Norbury, C.C.; Malide, D.; Gibbs, J.S.; Bennink, J.R.; Yewdell, J.W. Visualizing Priming of Virus-Specific CD8+ T Cells by Infected Dendritic Cells in Vivo. Nat. Immunol. 2002, 3, 265–271.
- Pollara, G.; Kwan, A.; Newton, P.J.; Handley, M.E.; Chain, B.M.; Katz, D.R. Dendritic Cells in Viral Pathogenesis: Protective or Defective? Int. J. Exp. Pathol. 2005, 86, 187–204.
- Finlay, B.B.; McFadden, G. Anti-Immunology: Evasion of the Host Immune System by Bacterial and Viral Pathogens. Cell 2006, 124, 767–782.
- Rescigno, M. Dendritic Cell Functions: Learning from Microbial Evasion Strategies. Semin. Immunol. 2015, 27, 119–124.
- Cameron, P.U.; Freudenthal, P.S.; Barker, J.M.; Gezelter, S.; Inaba, K.; Steinman, R.M. Dendritic Cells Exposed to Human Immunodeficiency Virus Type-1 Transmit a Vigorous Cytopathic Infection to CD4+ T Cells. Science 1992, 257, 383–387.
- Pope, M.; Betjes, M.G.H.; Romani, N.; Hirmand, H.; Cameron, P.U.; Hoffman, L.; Gezelter, S.; Schuler, G.; Steinman, R.M. Conjugates of Dendritic Cells and Memory T Lymphocytes from Skin Facilitate Productive Infection with HIV-1. Cell 1994, 78, 389–398.
- Lee, B.; Sharron, M.; Montaner, L.J.; Weissman, D.; Doms, R.W. Quantification of CD4, CCR5, and CXCR4 Levels on Lymphocyte Subsets, Dendritic Cells, and Differentially Conditioned Monocyte-Derived Macrophages. Proc. Natl. Acad. Sci. USA 1999, 96, 5215–5220.
- Turville, S.G.; Santos, J.J.; Frank, I.; Cameron, P.U.; Wilkinson, J.; Miranda-Saksena, M.; Dable, J.; Stössel, H.; Romani, N.; Piatak, M.; et al. Immunodeficiency Virus Uptake, Turnover, and 2-Phase Transfer in Human Dendritic Cells. Blood 2004, 103, 2170–2179.
- Moris, A.; Nobile, C.; Buseyne, F.; Porrot, F.; Abastado, J.P.; Schwartz, O. DC-SIGN Promotes Exogenous MHC-I-Restricted HIV-1 Antigen Presentation. Blood 2004, 103, 2648–2654.
- Laguette, N.; Sobhian, B.; Casartelli, N.; Ringeard, M.; Chable-Bessia, C.; Ségéral, E.; Yatim, A.; Emiliani, S.; Schwartz, O.; Benkirane, M. SAMHD1 Is the Dendritic- and Myeloid-Cell-Specific HIV-1 Restriction Factor Counteracted by Vpx. Nature 2011, 474, 654–657.
- Hrecka, K.; Hao, C.; Gierszewska, M.; Swanson, S.K.; Kesik-Brodacka, M.; Srivastava, S.; Florens, L.; Washburn, M.P.; Skowronski, J. Vpx Relieves Inhibition of HIV-1 Infection of Macrophages Mediated by the SAMHD1 Protein. Nature 2011, 474, 658–661.
- Lahouassa, H.; Daddacha, W.; Hofmann, H.; Ayinde, D.; Logue, E.C.; Dragin, L.; Bloch, N.; Maudet, C.; Bertrand, M.; Gramberg, T.; et al. SAMHD1 Restricts the Replication of Human Immunodeficiency Virus Type 1 by Depleting the Intracellular Pool of Deoxynucleoside Triphosphates. Nat. Immunol. 2012, 13, 223–228.
- Sheehy, A.M.; Gaddis, N.C.; Choi, J.D.; Malim, M.H. Isolation of a Human Gene That Inhibits HIV-1 Infection and Is Suppressed by the Viral Vif Protein. Nature 2002, 418, 646–650.
- Mariani, R.; Chen, D.; Schröfelbauer, B.; Navarro, F.; König, R.; Bollman, B.; Münk, C.; Nymark-McMahon, H.; Landau, N.R. Species-Specific Exclusion of APOBEC3G from HIV-1 Virions by Vif. Cell 2003, 114, 21–31.
- Mangeat, B.; Turelli, P.; Caron, G.; Friedli, M.; Perrin, L.; Trono, D. Broad Antiretroviral Defence by Human APOBEC3G through Lethal Editing of Nascent Reverse Transcripts. Nature 2003, 424, 99–103.
- Geijtenbeek, T.B.H.; Tornsma, R.; van Vliet, S.J.; van Duijnhoven, G.C.F.; Adema, G.J.; van Kooyk, Y.; Figdor, C.G. Identification of DC-SIGN, a Novel Dendritic Cell-Specific ICAM-3 Receptor That Supports Primary Immune Responses. Cell 2000, 100, 575–585.
- Turville, S.G.; Cameron, P.U.; Handley, A.; Lin, G.; Pöhlmann, S.; Doms, R.W.; Cunningham, A.L. Diversity of Receptors Binding HIV on Dendritic Cell Subsets. Nat. Immunol. 2002, 3, 975–983.
- Wu, L.; Bashirova, A.A.; Martin, T.D.; Villamide, L.; Mehlhop, E.; Chertov, A.O.; Unutmaz, D.; Pope, M.; Carrington, M.; KewalRamani, V.N. Rhesus Macaque Dendritic Cells Efficiently Transmit Primate Lentiviruses Independently of DC-SIGN. Proc. Natl. Acad. Sci. USA 2002, 99, 1568–1573.
- Gummuluru, S.; Rogel, M.; Stamatatos, L.; Emerman, M. Binding of Human Immunodeficiency Virus Type 1 to Immature Dendritic Cells Can Occur Independently of DC-SIGN and Mannose Binding C-Type Lectin Receptors via a Cholesterol-Dependent Pathway. J. Virol. 2003, 77, 12865–12874.
- Trumpfheller, C.; Park, C.G.; Finke, J.; Steinman, R.M.; Granelli-Piperno, A. Cell Type-Dependent Retention and Transmission of HIV-1 by DC-SIGN. Int. Immunol. 2003, 15, 289–298.
- Granelli-Piperno, A.; Pritsker, A.; Pack, M.; Shimeliovich, I.; Arrighi, J.-F.; Park, C.G.; Trumpfheller, C.; Piguet, V.; Moran, T.M.; Steinman, R.M. Dendritic Cell-Specific Intercellular Adhesion Molecule 3-Grabbing Nonintegrin/CD209 Is Abundant on Macrophages in the Normal Human Lymph Node and Is Not Required for Dendritic Cell Stimulation of the Mixed Leukocyte Reaction. J. Immunol. 2005, 175, 4265–4273.
- Boggiano, C.; Manel, N.; Littman, D.R. Dendritic Cell-Mediated Trans-Enhancement of Human Immunodeficiency Virus Type 1 Infectivity Is Independent of DC-SIGN. J. Virol. 2007, 81, 2519–2523.
- Wang, J.-H.; Janas, A.M.; Olson, W.J.; Wu, L. Functionally Distinct Transmission of Human Immunodeficiency Virus Type 1 Mediated by Immature and Mature Dendritic Cells. J. Virol. 2007, 81, 8933–8943.
- Izquierdo-Useros, N.; Blanco, J.; Erkizia, I.; Fernández-Figueras, M.T.; Borràs, F.E.; Naranjo-Gómez, M.; Bofill, M.; Ruiz, L.; Clotet, B.; Martinez-Picado, J. Maturation of Blood-Derived Dendritic Cells Enhances Human Immunodeficiency Virus Type 1 Capture and Transmission. J. Virol. 2007, 81, 7559–7570.
- Izquierdo-Useros, N.; Lorizate, M.; Puertas, M.C.; Rodriguez-Plata, M.T.; Zangger, N.; Erikson, E.; Pino, M.; Erkizia, I.; Glass, B.; Clotet, B.; et al. Siglec-1 Is a Novel Dendritic Cell Receptor That Mediates HIV-1 Trans-Infection through Recognition of Viral Membrane Gangliosides. PLoS Biol. 2012, 10, e1001448.
- Puryear, W.B.; Akiyama, H.; Geer, S.D.; Ramirez, N.P.; Yu, X.; Reinhard, B.M.; Gummuluru, S. Interferon-Inducible Mechanism of Dendritic Cell-Mediated HIV-1 Dissemination Is Dependent on Siglec-1/CD169. PLoS Pathog. 2013, 9, e1003291.
- Pino, M.; Erkizia, I.; Benet, S.; Erikson, E.; Fernández-Figueras, M.T.; Guerrero, D.; Dalmau, J.; Ouchi, D.; Rausell, A.; Ciuffi, A.; et al. HIV-1 Immune Activation Induces Siglec-1 Expression and Enhances Viral Trans-Infection in Blood and Tissue Myeloid Cells. Retrovirology 2015, 12, 37.
- Crocker, P.R.; Gordon, S. Mouse Macrophage Hemagglutinin (Sheep Erythrocyte Receptor) with Specificity for Sialylated Glycoconjugates Characterized by a Monoclonal Antibody. J. Exp. Med. 1989, 169, 1333–1346.
- Rempel, H.; Calosing, C.; Sun, B.; Pulliam, L. Sialoadhesin Expressed on IFN-Induced Monocytes Binds HIV-1 and Enhances Infectivity. PLoS ONE 2008, 3, e1967.
- Crocker, P.R.; Mucklow, S.; Bouckson, V.; McWilliam, A.; Willis, A.C.; Gordon, S.; Milon, G.; Kelm, S.; Bradfield, P. Sialoadhesin, a Macrophage Sialic Acid Binding Receptor for Haemopoietic Cells with 17 Immunoglobulin-like Domains. EMBO J. 1994, 13, 4490–4503.
- Crocker, P.R.; Vinson, M.; Kelm, S.; Drickamer, K. Molecular Analysis of Sialoside Binding to Sialoadhesin by NMR and Site-Directed Mutagenesis. Biochem. J. 1999, 341, 355–361.
- Hartnell, A.; Steel, J.; Turley, H.; Jones, M.; Jackson, D.G.; Crocker, P.R. Characterization of Human Sialoadhesin, a Sialic Acid Binding Receptor Expressed by Resident and Inflammatory Macrophage Populations. Blood 2001, 97, 288–296.
- Izquierdo-Useros, N.; Lorizate, M.; Contreras, F.-X.; Rodriguez-Plata, M.T.; Glass, B.; Erkizia, I.; Prado, J.G.; Casas, J.; Fabriàs, G.; Kräusslich, H.-G.; et al. Sialyllactose in Viral Membrane Gangliosides Is a Novel Molecular Recognition Pattern for Mature Dendritic Cell Capture of HIV-1. PLoS Biol. 2012, 10, e1001315.
- Puryear, W.B.; Yu, X.; Ramirez, N.P.; Reinhard, B.M.; Gummuluru, S. HIV-1 Incorporation of Host-Cell-Derived Glycosphingolipid GM3 Allows for Capture by Mature Dendritic Cells. Proc. Natl. Acad. Sci. USA 2012, 109, 7475–7480.
- Rodriguez-Plata, M.T.; Puigdomènech, I.; Izquierdo-Useros, N.; Puertas, M.C.; Carrillo, J.; Erkizia, I.; Clotet, B.; Blanco, J.; Martinez-Picado, J. The Infectious Synapse Formed between Mature Dendritic Cells and CD4(+) T Cells Is Independent of the Presence of the HIV-1 Envelope Glycoprotein. Retrovirology 2013, 10, 42.
- McDonald, D.; Wu, L.; Bohks, S.M.; KewalRamani, V.N.; Unutmaz, D.; Hope, T.J. Recruitment of HIV and Its Receptors to Dendritic Cell-T Cell Junctions. Science 2003, 300, 1295–1297.
- Izquierdo-Useros, N.; Esteban, O.; Rodriguez-Plata, M.T.; Erkizia, I.; Prado, J.G.; Blanco, J.; García-Parajo, M.F.; Martinez-Picado, J. Dynamic Imaging of Cell-Free and Cell-Associated Viral Capture in Mature Dendritic Cells. Traffic 2011, 12, 1702–1713.
- Yu, H.J.; Reuter, M.A.; McDonald, D. HIV Traffics through a Specialized, Surface-Accessible Intracellular Compartment during Trans-Infection of T Cells by Mature Dendritic Cells. PLoS Pathog. 2008, 4, e1000134.
- Garcia, E.; Pion, M.; Pelchen-Matthews, A.; Collinson, L.; Arrighi, J.-F.F.; Blot, G.; Leuba, F.; Escola, J.-M.M.; Demaurex, N.; Marsh, M.; et al. HIV-1 Trafficking to the Dendritic Cell-T-Cell Infectious Synapse Uses a Pathway of Tetraspanin Sorting to the Immunological Synapse. Traffic 2005, 6, 488–501.
- Izquierdo-Useros, N.; Naranjo-Gómez, M.; Archer, J.; Hatch, S.C.; Erkizia, I.; Blanco, J.; Borràs, F.E.; Puertas, M.C.; Connor, J.H.; Fernádez-Figueras, M.T.; et al. Capture and Transfer of HIV-1 Particles by Mature Dendritic Cells Converges with the Exosome-Dissemination Pathway. Blood 2009, 113, 2732–2741.
- Théry, C.; Duban, L.; Segura, E.; Véron, P.; Lantz, O.; Amigorena, S. Indirect Activation of Naïve CD4+ T Cells by Dendritic Cell-Derived Exosomes. Nat. Immunol. 2002, 3, 1156–1162.
- Benet, S.; Gálvez, C.; Drobniewski, F.; Kontsevaya, I.; Arias, L.; Monguió-Tortajada, M.; Erkizia, I.; Urrea, V.; Ong, R.Y.; Luquin, M.; et al. Dissemination of Mycobacterium Tuberculosis Is Associated to a SIGLEC1 Null Variant That Limits Antigen Exchange via Trafficking Extracellular Vesicles. J. Extracell. Vesicles 2021, 10, e12046.
- Knight, S.C.; Patterson, S. Bone Marrow-Derived Dendritic Cells, Infection with Human Immunodeficiency Virus, and Immunopathology. Annu. Rev. Immunol. 1997, 15, 593–615.
- UNAIDS Data 2021. Available online: https://www.unaids.org/sites/default/files/media_asset/JC3032_AIDS_Data_book_2021_En.pdf (accessed on 10 November 2021).
- Spira, A.I.; Marx, P.A.; Patterson, B.K.; Mahoney, J.; Koup, R.A.; Wolinsky, S.M.; Ho, D.D. Cellular Targets of Infection and Route of Viral Dissemination after an Intravaginal Inoculation of Simian Immunodeficiency Virus into Rhesus Macaques. J. Exp. Med. 1996, 183, 215–225.
- Miller, C.J.; Li, Q.; Abel, K.; Kim, E.-Y.; Ma, Z.-M.; Wietgrefe, S.; La Franco-Scheuch, L.; Compton, L.; Duan, L.; Shore, M.D.; et al. Propagation and Dissemination of Infection after Vaginal Transmission of Simian Immunodeficiency Virus. J. Virol. 2005, 79, 9217–9227.
- Masurier, C.; Salomon, B.; Guettari, N.; Pioche, C.; Guigon, M.; Klatzmann, D.; Lachapelle, F. Dendritic Cells Route Human Immunodeficiency Virus to Lymph Nodes after Vaginal or Intravenous Administration to Mice. J. Virol. 1998, 72, 7822–7829.
- Hu, J.; Gardner, M.B.; Miller, C.J. Simian Immunodeficiency Virus Rapidly Penetrates the Cervicovaginal Mucosa after Intravaginal Inoculation and Infects Intraepithelial Dendritic Cells. J. Virol. 2000, 74, 6087–6095.
- Shen, R.; Kappes, J.C.; Smythies, L.E.; Richter, H.E.; Novak, L.; Smith, P.D. Vaginal Myeloid Dendritic Cells Transmit Founder HIV-1. J. Virol. 2014, 88, 7683–7688.
- Trifonova, R.T.; Bollman, B.; Barteneva, N.S.; Lieberman, J. Myeloid Cells in Intact Human Cervical Explants Capture HIV and Can Transmit It to CD4 T Cells. Front. Immunol. 2018, 9, 2719.
- Hu, Q.; Frank, I.; Williams, V.; Santos, J.J.; Watts, P.; Griffin, G.E.; Moore, J.P.; Pope, M.; Shattock, R.J. Blockade of Attachment and Fusion Receptors Inhibits HIV-1 Infection of Human Cervical Tissue. J. Exp. Med. 2004, 199, 1065–1075.
- Sanders, R.W.; de Jong, E.C.; Baldwin, C.E.; Schuitemaker, J.H.N.; Kapsenberg, M.L.; Berkhout, B. Differential Transmission of Human Immunodeficiency Virus Type 1 by Distinct Subsets of Effector Dendritic Cells. J. Virol. 2002, 76, 7812–7821.
- Perez-Zsolt, D.; Cantero-Pérez, J.; Erkizia, I.; Benet, S.; Pino, M.; Serra-Peinado, C.; Hernández-Gallego, A.; Castellví, J.; Tapia, G.; Arnau-Saz, V.; et al. Dendritic Cells from the Cervical Mucosa Capture and Transfer HIV-1 via Siglec-1. Front. Immunol. 2019, 10, 825.
