Lithium/sulfur (Li/S) cells that offer an ultrahigh theoretical specific energy of 2600 Wh/kg are considered one of the most promising next-generation rechargeable battery systems for the electrification of transportation. This review introduces electrode manufacturing and modeling methodologies to overcome the current technical issues. The prospects for rational modeling and manufacturing strategies are discussed, to establish a new design standard for Li/S batteries.
- lithium/sulfur cells
- computational modeling
- sulfur electrode
- electrolyte to sulfur ratio
- high specific energy
1. Introduction
Li/S batteries possess exceptional specific energy and a standard open-circuit potential of 2.15 V [14][1]. The theoretical maximum specific energy of a Li/S battery is 2600 W h kg S−1 [15][2], assuming the sulfur is fully utilized. However, complete utilization is not technically feasible due to the sluggish reaction kinetics and the insulating properties of elemental sulfur [16,17][3][4]. In addition, the weight of other cell components such as current collectors, separator, electrolyte, and cell housing materials should be considered when estimating the actual specific energy of Li/S batteries. Considering practical cell design parameters, a more realistic projection estimates the specific energy of Li/S batteries at between 400 and 500 W h kg−1 [12,18][5][6]. Though still early in the research phase of development, this technology currently exceeds the peak specific energy of Li-ion cells by about 100 W h kg−1 [12,19][5][7]. Li/S batteries also show improved safety during operation under abuse conditions compared to LiBs. Nail penetration tests demonstrated no explosive energy release when punctured due to a solid insulating coating that forms from lithium polysulfide (Li-PS), thereby preventing an internal short circuit via the penetrator [20][8]. Furthermore, because of the chemical stability of the active materials in the completely discharged state, the cells can be stored indefinitely without experiencing damage and without hazard risk [21][9].
2. Challenges in the Design and Development of Li/S Cell Components
2.1. Sulfur Electrode Design Challenges
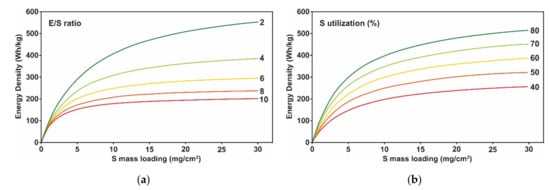
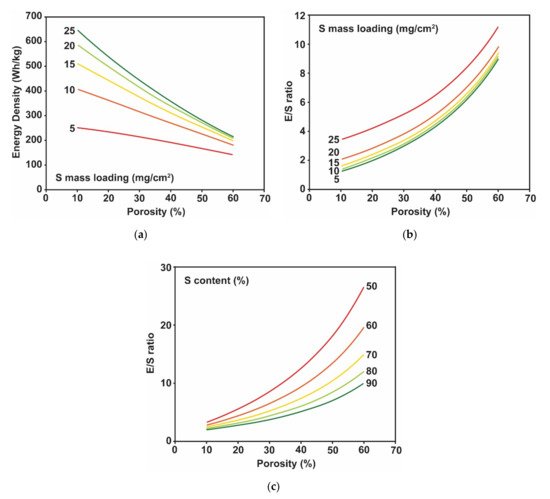
2.2. Challenges in Lithium Metal Electrode
2.3. Electrolyte Design Challenges
2.4. Separator Design Challenges
References
- Fang, R.; Zhao, S.; Sun, Z.; Wang, D.-W.; Cheng, H.-M.; Li, F. More Reliable Lithium-Sulfur Batteries: Status, Solutions and Prospects. Adv. Mater. 2017, 29, 1606823.
- Bruce, P.G.; Freunberger, S.A.; Hardwick, L.J.; Tarascon, J.-M. Li-O2 and Li-S batteries with high energy storage. Nat. Mater. 2012, 11, 19–29.
- Fotouhi, A.; Auger, D.J.; O’Neill, L.; Cleaver, T.; Walus, S. Lithium-Sulfur Battery Technology Readiness and Applications—A Review. Energies 2017, 10, 1937.
- Peng, H.-J.; Huang, J.-Q.; Cheng, X.-B.; Zhang, Q. Review on High-Loading and High-Energy Lithium–Sulfur Batteries. Adv. Energy Mater. 2017, 7, 1700260.
- Robinson, J.; Xi, K.; Kumar, R.V.; Ferrari, A.C.; Au, H.; Titirici, M.-M.; Puerto, A.P.; Kucernak, A.; Fitch, S.D.S.; Garcia-Araez, N.; et al. 2021 Roadmap on Lithium Sulfur Batteries. J. Phys. Energy 2021, 3, 031501.
- Li, C.; Zhang, H.; Otaegui, L.; Singh, G.; Armand, M.; Rodriguez-Martinez, L.M. Estimation of energy density of Li-S batteries with liquid and solid electrolytes. J. Power Sources 2016, 326, 1–5.
- Durmus, Y.E.; Zhang, H.; Baakes, F.; Desmaizieres, G.; Hayun, H.; Yang, L.; Kolek, M.; Küpers, V.; Janek, J.; Mandler, D.; et al. Side by Side Battery Technologies with Lithium-Ion Based Batteries. Adv. Energy Mater. 2020, 10, 2000089.
- Huang, X.; Xue, J.; Xiao, M.; Wang, S.; Li, Y.; Zhang, S.; Meng, Y. Comprehensive evaluation of safety performance and failure mechanism analysis for lithium sulfur pouch cells. Energy Storage Mater. 2020, 30, 87–97.
- Manthiram, A.; Fu, Y.; Chung, S.-H.; Zu, C.; Su, Y.-S. Rechargeable Lithium–Sulfur Batteries. Chem. Rev. 2014, 114, 11751–11787.
- Fan, F.Y.; Woodford, W.H.; Li, Z.; Baram, N.; Smith, K.C.; Helal, A.; McKinley, G.H.; Carter, W.C.; Chiang, Y.-M. Polysulfide Flow Batteries Enabled by Percolating Nanoscale Conductor Networks. Nano Lett. 2014, 14, 2210–2218.
- Hwa, Y.; Cairns, E.J. Nanostructured Sulfur and Sulfides for Advanced Lithium/Sulfur Cells. ChemElectroChem 2020, 7, 3927–3942.
- Liang, J.; Sun, Z.-H.; Li, F.; Cheng, H.-M. Carbon materials for Li–S batteries: Functional evolution and performance improvement. Energy Storage Mater. 2016, 2, 76–106.
- Eftekhari, A.; Kim, D.-W. Cathode materials for lithium–sulfur batteries: A practical perspective. J. Mater. Chem. A 2017, 5, 17734–17776.
- Huang, L.; Li, J.; Liu, B.; Li, Y.; Shen, S.; Deng, S.; Lu, C.; Zhang, W.; Xia, Y.; Pan, G.; et al. Electrode Design for Lithium–Sulfur Batteries: Problems and Solutions. Adv. Funct. Mater. 2020, 30, 1910375.
- Hagen, M.; Fanz, P.; Tübke, J. Cell energy density and electrolyte/sulfur ratio in Li–S cells. J. Power Sources 2014, 264, 30–34.
- Mikhaylik, Y.V.; Kovalev, I.; Schock, R.; Kumaresan, K.; Xu, J.; Affinito, J. High Energy Rechargeable Li-S Cells for EV Application: Status, Remaining Problems and Solutions. ECS Trans. 2010, 25, 23.
- Dörfler, S.; Althues, H.; Härtel, P.; Abendroth, T.; Schumm, B.; Kaskel, S. Challenges and Key Parameters of Lithium-Sulfur Batteries on Pouch Cell Level. Joule 2020, 4, 539–554.
- Bhargav, A.; He, J.; Gupta, A.; Manthiram, A. Lithium-Sulfur Batteries: Attaining the Critical Metrics. Joule 2020, 4, 285–291.
- Hwa, Y.; Kim, H.W.; Shen, H.; Parkinson, D.Y.; McCloskey, B.D.; Cairns, E.J. A sustainable sulfur–carbonaceous composite electrode toward high specific energy rechargeable cells. Mater. Horiz. 2020, 7, 524–529.
- Lv, D.; Zheng, J.; Li, Q.; Xie, X.; Ferrara, S.; Nie, Z.; Mehdi, L.B.; Browning, N.D.; Zhang, J.-G.; Graff, G.L.; et al. High Energy Density Lithium–Sulfur Batteries: Challenges of Thick Sulfur Cathodes. Adv. Energy Mater. 2015, 5, 1402290.
- Chung, S.-H.; Manthiram, A. Designing Lithium-Sulfur Cells with Practically Necessary Parameters. Joule 2018, 2, 710–724.
- Li, M.; Zhang, Y.; Bai, Z.; Liu, W.W.; Liu, T.; Gim, J.; Jiang, G.; Yuan, Y.; Luo, D.; Feng, K.; et al. A Lithium–Sulfur Battery using a 2D Current Collector Architecture with a Large-Sized Sulfur Host Operated under High Areal Loading and Low E/S Ratio. Adv. Mater. 2018, 30, 1804271.
- Wang, H.; Liu, Y.; Li, Y.; Cui, Y. Lithium Metal Anode Materials Design: Interphase and Host. Electrochem. Energy Rev. 2019, 2, 509–517.
- Aurbach, D.; Zinigrad, E.; Cohen, Y.; Teller, H. A short review of failure mechanisms of lithium metal and lithiated graphite anodes in liquid electrolyte solutions. Solid State Ion. 2002, 148, 405–416.
- Chen, X.; Hou, T.; Persson, K.A.; Zhang, Q. Combining theory and experiment in lithium–sulfur batteries: Current progress and future perspectives. Mater. Today 2019, 22, 142–158.
- Park, M.S.; Ma, S.B.; Lee, D.J.; Im, D.; Doo, S.-G.; Yamamoto, O. A Highly Reversible Lithium Metal Anode. Sci. Rep. 2014, 4, 3815.
- Schmuch, R.; Wagner, R.; Hörpel, G.; Placke, T.; Winter, M. Performance and cost of materials for lithium-based rechargeable automotive batteries. Nat. Energy 2018, 3, 267–278.
- Ye, Y.; Song, M.-K.; Xu, Y.; Nie, K.; Liu, Y.; Feng, J.; Sun, X.; Cairns, E.J.; Zhang, Y.; Guo, J. Lithium nitrate: A double-edged sword in the rechargeable lithium-sulfur cell. Energy Storage Mater. 2019, 16, 498–504.
- Qu, C.; Chen, Y.; Yang, X.; Zhang, H.; Li, X.; Zhang, H. LiNO3-free electrolyte for Li-S battery: A solvent of choice with low Ksp of polysulfide and low dendrite of lithium. Nano Energy 2017, 39, 262–272.
- Suo, L.; Hu, Y.-S.; Li, H.; Armand, M.; Chen, L. A new class of Solvent-in-Salt electrolyte for high-energy rechargeable metallic lithium batteries. Nat. Commun. 2013, 4, 1481.
- Miller, E.C.; Toney, M.F. X-Ray Studies of Energy Materials. In Synchrotron Light Sources and Free-Electron Lasers: Accelerator Physics, Instrumentation and Science Applications; Jaeschke, E.J., Khan, S., Schneider, J.R., Hastings, J.B., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 1803–1824. ISBN 978-3-030-23201-6.
- Weber, R.; Genovese, M.; Louli, A.J.; Hames, S.; Martin, C.; Hill, I.G.; Dahn, J.R. Long cycle life and dendrite-free lithium morphology in anode-free lithium pouch cells enabled by a dual-salt liquid electrolyte. Nat. Energy 2019, 4, 683–689.
- Yue, J.; Yan, M.; Yin, Y.-X.; Guo, Y.-G. Progress of the Interface Design in All-Solid-State Li–S Batteries. Adv. Funct. Mater. 2018, 28, 1707533.
- Lei, D.; Shi, K.; Ye, H.; Wan, Z.; Wang, Y.; Shen, L.; Li, B.; Yang, Q.-H.; Kang, F.; He, Y.-B. Solid-State Electrolytes: Progress and Perspective of Solid-State Lithium–Sulfur Batteries (Adv. Funct. Mater. 38/2018). Adv. Funct. Mater. 2018, 28, 1870272.
- Umeshbabu, E.; Zheng, B.; Yang, Y. Recent Progress in All-Solid-State Lithium–Sulfur Batteries Using High Li-Ion Conductive Solid Electrolytes. Electrochem. Energy Rev. 2019, 2, 199–230.
- Deimede, D.; Elmasides, C. Separators for Lithium-Ion Batteries: A Review on the Production Processes and Recent Developments. Energy Technol. 2015, 3, 453–468.
- Xiang, Y.; Li, J.; Lei, J.; Liu, D.; Xie, Z.; Qu, D.; Li, K.; Deng, T.; Tang, H. Advanced Separators for Lithium-Ion and Lithium–Sulfur Batteries: A Review of Recent Progress. ChemSusChem 2016, 9, 1–18.
- Deng, N.; Kang, W.; Liu, Y.; Ju, J.; Wu, D.; Lia, L.; Hassan, B.S.; Cheng, B. A review on separators for lithium–sulfur battery: Progress and prospects. J. Power Sources 2016, 331, 132–155.
- Wood, D.L.; Li, J.; Daniel, C. Prospects for reducing the processing cost of lithium ion batteries. J. Power Sources 2015, 275, 234–242.
