COVID-19 is an inflammatory disease caused by SARS-CoV-2 and can manifest as various symptoms ranging from mild symptoms or asymptomatic cases to severe pneumonia that can progress to acute respiratory distress syndrome (ARDS) and death. AThe entry describes a study assessinged the utility of various inflammatory markers in predicting mortality among hospitalized patients with COVID-19.
- COVID-19
- mortality
- prediction
1. Introduction
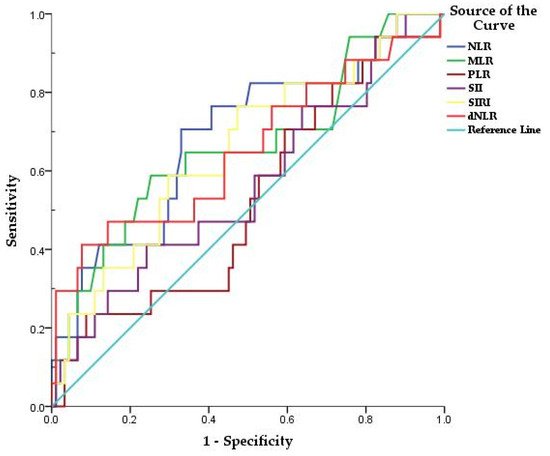
COVID-19 has a three-phase progression: initial disease caused by active infection, a second pulmonary phase, and, when severe, a third phase described by hyper-inflammation, cytokine storm, elevated biomarker levels of cardiac injury, and significant morbidity and mortality [1]. Serum biochemical analysis and blood count analysis are commonly used blood tests, which could be faster, easier to use, and low-cost techniques that can facilitate the diagnosis and prognosis of this disease [2]. From these routine tests, inflammatory markers have been used for predicting the severity of COVID-19 such as neutrophil to lymphocyte ratio (NLR), derivate neutrophil to lymphocyte ratio (dNLR), monocyte to lymphocyte ratio (MLR), and platelet to lymphocyte ratio (PLR) [2][3]. NLR and PLR are biomarkers reflecting systemic inflammation, neutrophil and platelet activation, and are associated with increased mortality in cardiovascular disease and poor prognosis in various cancers or in polycythemia vera [4][5][6]. In addition, a higher NLR, and decreased PLR were predictive of poor survival in patients with myelofibrosis [7]. Derived neutrophil-to-lymphocyte ratio (dNLR) is a potential new biomarker for systemic inflammation, defined as the absolute neutrophil count (ANC)/white cell count (WBC)—absolute neutrophil count (ANC), and has prognostic value in patients with several types of cancer [8][9][10]. Unlike NLR, dNLR includes monocytes and other granulocytes by using the difference between WBC and neutrophils in the denominator. Poorly differentiated and immature neutrophils can be released into a pro-inflammatory environment, which rapidly increases neutrophil generation, thus dNLR is likely to reflect this negative inflammation more comprehensively [8][10]. The systemic inflammatory response index (SIRI) may also reflect the host’s immune and inflammatory balance [11]. In addition, systemic immune-inflammation index (SII), defined as platelet count × NLR, is effective in reflecting inflammatory status, being a basic biomarker for predicting the prognosis [3]. The current study assesses the utility of various inflammatory markers in predicting mortality among hospitalized patients with COVID-19.
2. Participants Characteristics
| Total | Survivors 91 (84.3%) |
Deaths 17 (15.7%) |
p Value |
|---|
| Variables | Area | Std. Error | Asymptotic Sig. | 95% Confidence Interval |
Sensitivity | Sensibility | Cut-Off | |
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| Age (Mean ± SD) |
63.31 ± 14.83 | 62.02 ± 14.73 | 70.18 ± 13.83 | 0.03 | ||||
| Variables | Adjusted OR * | p Value | ||||||
|---|---|---|---|---|---|---|---|---|
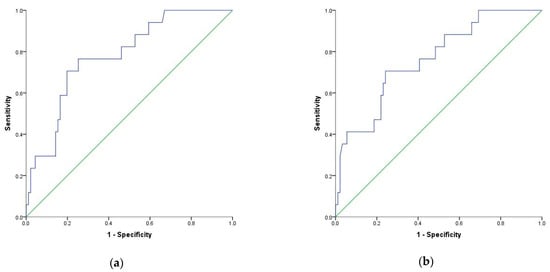
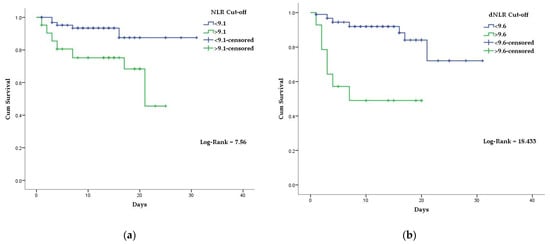
| NLR | 0.689 | 0.074 | 0.014 | 0.544 | 0.833 | 70% | 67% | 9.1 |
| Comorbidities No. (%) |
||||||||
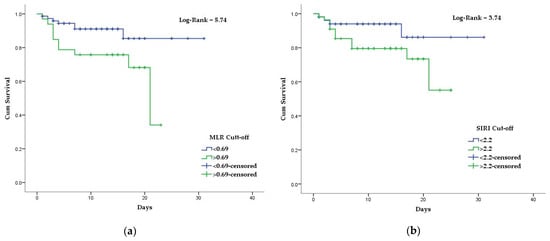
| MLR | 0.661 | 0.078 | 0.036 | 0.508 | 0.813 | 58% | 74% |
| Variables | HR (95%CI) | p Value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| NLR | 3.85 (1.35–10.95) | 0.01 | |||||||
| 0.69 | |||||||||
| dNLR | |||||||||
| NLR | 4.14 | 0.002 | |||||||
| 6.4 (2.40–17.18) | |||||||||
| dNLR | 14.09 | 0.001 | Diabetes | 50 (46.3%) | 40 (44.0%) | 10 (58.8%) | 0.29 | ||
| <0.001 | SIRI | 0.655 | 0.074 | 0.042 | 0.511 | 0.800 | 76% | 52% | 2.2 |
| MLR | 3.05 (1.16–8.05) | Hypertension | |||||||
| MLR | 3.29 | 76 (70.4%) | 62 (68.1%) | 14 (82.4%) | 0.38 | ||||
| 0.02 | 0.04 | Heart diseases | 51 (47.2%) | 38 (41.8%) | 13 (76.5%) | 0.01 | |||
| Chronic lung diseases | 23 (21.3%) | 17 (18.7%) | 6 (35.3%) | ||||||
| SIRI | 0.19 | ||||||||
| Complete blood count (Mean ± SD) |
|||||||||
| 3.06 | White blood cell (×1012/L) | 8.71 ± 5.74 | 8.71 ± 5.76 | 8.73 ± 5.81 | 0.98 | ||||
| Neutrophil count (×109/L) | 6.96 ± 4.36 | 6.75 ± 4.18 | 8.06 ± 5.19 | 0.25 | |||||
| Lymphocyte count (×109/L) | 0.98 ± 0.78 | 1.03 ± 0.82 | 0.73 ± 0.44 | 0.03 | |||||
| 0.08 | Monocyte count (×109/L) | 0.47 ± 0.32 | 0.47 ± 0.32 | 0.51 ± 0.33 | 0.64 | ||||
| Hemoglobin (g/dL) |
13.15 ± 1.78 | 13.27 ± 1.64 | 12.50 ± 2.36 | 0.10 | |||||
| Platelet count (×109/L) |
242 ± 109 | 252 ± 112 | 192 ± 79 | 0.03 | |||||
| Inflammatory markers | |||||||||
| NLR | 9.18 ± 6.7 | 8.31 ± 5.74 | 13.83 ± 9.23 | 0.001 | |||||
| MLR | 0.58 ±0.44 | 0.53 ± 0.39 | 0.83 ± 0.59 | 0.01 | |||||
| PLR | 327 ± 72 | 324 ± 219 | 345 ± 235 | 0.71 | |||||
| dNLR | 5.16 ± 3.76 | 4.77 ± 3.45 | 7.07 ± 4.64 | 0.01 | |||||
| SII | 2280 ± 1950 | 2183 ± 1847 | 2798.± 2429 | 0.23 | |||||
| SIRI | 4.57 ± 5.12 | 4.11 ± 4.67 | 7.02 ± 6.72 | 0.03 | |||||
| O2 Saturation * | 91.96 ± 6.16 | 92.26 ± 5.96 | 90.35 ± 7.13 | 0.24 | |||||
| Hospitalization length | 11.89 (6.56) | 12.96 | 6.18 | <0.001 |
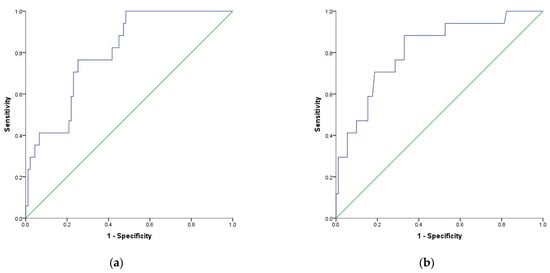
3. Using Optimal Cut-Off Values of Inflammatory Markers to Predict Mortality in Patients with COVID-19
| dNLR |
| 0.652 | |||||
| 0.082 | |||||
| 0.047 | 0.491 | 0.813 | 41% | 92% | 9.6 |
4. Association of Inflammatory Biomarkers Results with The COVID-19 Mortality
References
- Koupenova, M.; Freedman, J.E. Platelets and COVID-19: Inflammation, Hyperactivation and Additional Questions. Circ. Res. 2020, 127, 1419–1421.
- Yang, A.-P.; Liu, J.; Tao, W.; Li, H. The Diagnostic and Predictive Role of NLR, d-NLR and PLR in COVID-19 Patients. Int. Immunopharmacol. 2020, 84, 106504.
- Karimi, A.; Shobeiri, P.; Kulasinghe, A.; Rezaei, N. Novel Systemic Inflammation Markers to Predict COVID-19 Prognosis. Front. Immunol. 2021, 12, 741061.
- Hirahara, T.; Arigami, T.; Yanagita, S.; Matsushita, D.; Uchikado, Y.; Kita, Y.; Mori, S.; Sasaki, K.; Omoto, I.; Kurahara, H.; et al. Combined Neutrophil-Lymphocyte Ratio and Platelet-Lymphocyte Ratio Predicts Chemotherapy Response and Prognosis in Patients with Advanced Gastric Cancer. BMC Cancer 2019, 19, 672.
- Zhang, Y.; Lu, J.-J.; Du, Y.-P.; Feng, C.-X.; Wang, L.-Q.; Chen, M.-B. Prognostic Value of Neutrophil-to-Lymphocyte Ratio and Platelet-to-Lymphocyte Ratio in Gastric Cancer. Medicine 2018, 97, e0144.
- Krečak, I.; Holik, H.; Morić Perić, M.; Zekanović, I.; Coha, B.; Valovičić Krečak, M.; Gverić-Krečak, V.; Lucijanić, M. Neutrophil-to-lymphocyte and Platelet-to-lymphocyte Ratios as Prognostic Biomarkers in Polycythemia Vera . Int. J. Lab. Hematol. 2021. Available online: https://onlinelibrary.wiley.com (accessed on 31 December 2021).
- Lucijanic, M.; Cicic, D.; Stoos-Veic, T.; Pejsa, V.; Lucijanic, J.; Fazlic Dzankic, A.; Vlasac Glasnovic, J.; Soric, E.; Skelin, M.; Kusec, R. Elevated Neutrophil-to-Lymphocyte-Ratio and Platelet-to-Lymphocyte Ratio in Myelofibrosis: Inflammatory Biomarkers or Representatives of Myeloproliferation Itself? Anticancer Res. 2018, 38, 3157–3163.
- Proctor, M.J.; McMillan, D.C.; Morrison, D.S.; Fletcher, C.D.; Horgan, P.G.; Clarke, S.J. A Derived Neutrophil to Lymphocyte Ratio Predicts Survival in Patients with Cancer. Br. J. Cancer 2012, 107, 695–699.
- Grenader, T.; Nash, S.; Adams, R.; Kaplan, R.; Fisher, D.; Maughan, T.; Bridgewater, J. Derived Neutrophil Lymphocyte Ratio Is Predictive of Survival from Intermittent Therapy in Advanced Colorectal Cancer: A Post Hoc Analysis of the MRC COIN Study. Br. J. Cancer 2016, 114, 612–615.
- Yang, T.; Hao, L.; Yang, X.; Luo, C.; Wang, G.; Cai, C.L.; Qi, S.; Li, Z. Prognostic Value of Derived Neutrophil-to-lymphocyte Ratio (DNLR) in Patients with Non-small Cell Lung Cancer Receiving Immune Checkpoint Inhibitors: A Meta-analysis. BMJ Open 2021, 11, e049123.
- Jiang, S.; Wang, S.; Wang, Q.; Deng, C.; Feng, Y.; Ma, F.; Ma, J.; Liu, X.; Hu, C.; Hou, T. Systemic Inflammation Response Index (SIRI) Independently Predicts Survival in Advanced Lung Adenocarcinoma Patients Treated with First-Generation EGFR-TKIs. Cancer Manag. Res. 2021, 13, 1315–1322.
