Diaper dermatitis is a common type of irritant contact dermatitis occurring in infants and toddlers. Its occurrence is triggered by an unfavorable environment under the diaper, damage to skin integrity by fecal enzyme degradation, overhydration and disruption of the lipid bilayer structure facilitating the entry of irritants and microorganisms. In diaper dermatitis development, the central proinflammatory cytokines are IL-1α, IL-8 and TNF-α. The initial release of IL-1α and TNF-α starts a further cascade of pro-inflammatory chemo- and cytokines, resulting in inflammation and erythema of the skin. A recently recognized factor in diaper dermatitis is the composition of the skin microbiome; common pathogenic strains Candida albicans and Staphylococcus aureus are associated with skin irritation. The resulting impaired microbiome composition produces a local inflammatory response and may thus worsen the initial dermatitis clinical presentation and subsequent healing.
- diaper dermatitis
- immunology
- inflammation
- microbiome
- nappy rash
- pediatrics
- pH
- probiotics
- skin
1. Introduction
2. Microbiome Composition and Skin Immunology in Diaper Dermatitis
2.1. Early Fetal and Postnatal Microbiome
2.2. The Microbiome in Diaper Dermatitis
2.3. Inflammation in Diaper Dermatitis
Skin inflammation is generally triggered by external factors such as allergen intake, contact with microbes or with irritants, UV radiation and by other, less well-defined stimuli [34][30]. In DD skin irritation is affected by adverse fecal enzymes, friction and subsequent skin maceration, high pH, the presence of urine and prolonged skin contact with feces [6]. Inadequate skin care, certain microbial invasion, antibiotic use and a lack of individual nutrients also play a role (Figure 1) [3]. The resulting inflammatory process involves a complex interplay of events between skin cells, immune cells, inflammatory cytokines, and chemokines [35][31].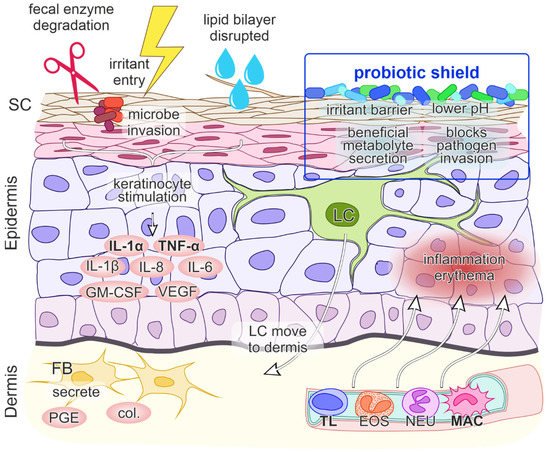
2.4. Role of Skin pH
2.5. Inflammatory Signaling
At a molecular level, when DD results from a contact etiology, irritant penetration through the skin induces endogenous “danger” signals, which cause direct damage to keratinocytes and the release of cytokines and chemokines IL-1α, IL-1β and TNF-α. These cytokines unsurprisingly play a central role in DD immune system activation, as they can trigger inflammation on their own. Keratinocytes additionally secrete the granulocyte-macrophage colony-stimulating factor (GM-CSF), IL-6 and IL-8, where IL-8 is a potent chemokine for lymphocytes and IL-6 influences the process of maturation of keratinocytes. The initial signal release is followed by the migration of Langerhans cells to the dermis, the production of collagenases and prostaglandin E by fibroblasts and the upregulation of intercellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule 1 (VCAM-1) on keratinocytes, fibroblasts and endothelial cells. Subsequently, blood vessels dilate and passage of inflammatory cells into the epidermis ensues, leading to inflammation and erythema (Figure 1) [35,46][31][35]. Less well characterized cytokines that are involved in ICD are CCL (chemokine (C-C motif) ligand) 20, CCL27, IL-10, IL-12 and IL-18 [35][31]. The cytokines that are primarily upregulated following irritant exposure are IL-1α and TNF-α [35,43,46][31][36][35]. Although the precise cytokines/chemokines activation cascade in DD is still unclear, IL-8 has also been specifically implicated as a strong contributor in DD [11,42][37][38].2.6. Probiotics as a “Protective Shield” against Skin Inflammation
Probiotics exert health effects on the skin directly through cutaneous formulations or indirectly through dietary supplementary formulations and intestinal microflora improvement [52][39]. Certain probiotics can modulate the cutaneous microflora, the lipid barrier, and the skin immune system, leading to the maintenance of skin homeostasis [53][40]. Beneficial dermal effects of probiotics have been shown via oral consummation through acting on the intestine with changes in systemic immune responses and thus immunomodulation of the skin, inhibition of allergen-induced tumors via changes in systemic immune responses and inhibition of harmful intestinal microflora. Probiotics can also act as antioxidant agents. Probiotics may be applied directly on the skin which then compete with harmful skin microflora, secrete useful metabolites, reduce pH and act as a barrier to harmful foreign environmental factors that are in contact with the skin (Figure 1) [52][39]. As the interest in the use of probiotics in DD is very recent, there is currently only very limited data available on their use as a DD prevention or treatment option. An older preliminary report from 1998 describes a trial of infant formula supplemented with Bifidobacterium lactis and Streptococcus thermophilus, which showed a small decrease in DD incidence compared to non-supplemented formula during the observation period [54][41]. Examining probiotics as a DD treatment, a single 2021 article favourably reports on a market research study that followed an at-home use of oral activated Bifidobacterium infantis EVC001 in infants with DD [55][42]. Although the consumer feedback was very positive, this type of research is methodologically limited and potentially biased. Consequently, more rigorous scientific research on use of probiotics in DD is desired.3. Conclusions and Outlook
References
- Benitez Ojeda, A.B.; Mendez, M.D. Diaper Dermatitis. In StatPearls Publishing. Updated 21 July 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK559067/ (accessed on 13 January 2021).
- Klunk, C.; Domingues, E.; Wiss, K. An update on diaper dermatitis. Clin. Dermatol. 2014, 32, 477–487.
- Tüzün, Y.; Wolf, R.; Bağlam, S.; Engin, B. Diaper (napkin) dermatitis: A fold (intertriginous) dermatosis. Clin. Dermatol. 2015, 33, 477–482.
- Blume-Peytavi, U.; Kanti, V. Prevention and treatment of diaper dermatitis. Pediatr. Dermatol. 2018, 35, s19–s23.
- Cohen, B. Differential diagnosis of diaper dermatitis. Clin. Pediatr. 2017, 56 (Suppl. 5), 16S–22S.
- Atherton, D.J. Understanding irritant napkin dermatitis. Int. J. Dermatol. 2016, 55 (Suppl. 1), 7–9.
- Sukhneewat, C.; Chaiyarit, J.; Techasatian, L. Diaper dermatitis: A survey of risk factors in Thai children aged under 24 months. BMC Dermatol. 2019, 19, 7.
- Schommer, N.N.; Gallo, R.L. Structure and function of the human skin microbiome. Trends Microbiol. 2013, 21, 660–668.
- Schneider, A.M.; Nelson, A.M. Skin microbiota: Friend or foe in pediatric skin health and skin disease. Pediatr. Dermatol. 2019, 36, 815–822.
- Schoch, J.J.; Monir, R.L.; Satcher, K.G.; Harris, J.; Triplett, E.; Neu, J. The infantile cutaneous microbiome: A review. Pediatr. Dermatol. 2019, 36, 574–580.
- Casterline, B.W.; Paller, A.S. Early development of the skin microbiome: Therapeutic opportunities. Pediatr. Res. 2021, 90, 731–737.
- Younge, N.; McCann, J.R.; Ballard, J.; Plunkett, C.; Akhtar, S.; Araújo-Pérez, F.; Murtha, A.; Brandon, D.; Seed, P.C. Fetal exposure to the maternal microbiota in humans and mice. JCI Insight 2019, 4, e127806.
- Krieger, Y.; Horev, A.; Wainstock, T.; Sheiner, E.; Walfisch, A. Meconium-stained amniotic fluid as a protective factor against childhood dermatitis and skin rash-related hospitalization in the offspring—A population-based cohort analysis. J. Eur. Acad Dermatol. Venereol. 2020, 34, 319–324.
- Dominguez-Bello, M.G.; Costello, E.K.; Contreras, M.; Magris, M.; Hidalgo, G.; Fierer, N.; Knight, R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl. Acad. Sci. USA 2010, 107, 11971–11975.
- Kennedy, E.A.; Connolly, J.; Hourihane, J.O.; Fallon, P.; McLean, W.I.; Murray, D.; Jo, J.-H.; Segre, J.A.; Kong, H.H.; Irvine, A.D. Skin microbiome before development of atopic dermatitis: Early colonization with commensal staphylococci at 2 months is associated with a lower risk of atopic dermatitis at 1 year. J. Allergy Clin. Immunol. 2017, 139, 166–172.
- Capone, K.A.; Dowd, S.E.; Stamatas, G.N.; Nikolovski, J. Diversity of the human skin microbiome early in life. J. Investig. Dermatol. 2011, 131, 2026–2032.
- Šikić Pogačar, M.; Maver, U.; Marčun Varda, N.; Mičetić-Turk, D. Diagnosis and management of diaper dermatitis in infants with emphasis on skin microbiota in the diaper area. Int. J. Dermatol. 2018, 57, 265–275.
- Grice, E.A.; Segre, J.A. The skin microbiome. Nat. Rev. Microbiol. 2011, 9, 244–253.
- Chu, D.M.; Ma, J.; Prince, A.L.; Antony, K.M.; Seferovic, M.D.; Aagaard, K.M. Maturation of the infant microbiome community structure and function across multiple body sites and in relation to mode of delivery. Nat. Med. 2017, 23, 314–326.
- Gaitanis, G.; Tsiouri, G.; Spyridonos, P.; Stefos, T.; Stamatas, G.N.; Velegraki, A.; Bassukas, I.D. Variation of cultured skin microbiota in mothers and their infants during the first year postpartum. Pediatr. Dermatol. 2019, 36, 460–465.
- Mattila-Sandholm, T.; Blum, S. Probiotics: Towards demonstrating efficacy. Trends Food Sci. Technol. 1999, 10, 393–399.
- Harmsen, H.J.M.; Wildeboer–Veloo, A.C.M.; Raangs, G.C.; Wagendorp, A.A.; Klijn, N.; Bindels, J.G.; Welling, G.W. Analysis of intestinal flora development in breast-fed and formula-fed infants by using molecular identification and detection methods. J. Pediatr. Gastroenterol. Nutr. 2000, 30, 61–67.
- Caramia, G.; Atzei, A.; Fanos, V. Probiotics and the skin. Clin. Dermatol. 2008, 26, 4–11.
- Mičetić Turk, D.; Turk, E.; Šikić Pogačar, M. Historical overview of breastfeeding in Slovenia. Acta Med.-Biotech. 2017, 10, 18–24.
- Kumbhare, S.V.; Patangia, D.V.; Patil, R.H.; Shouche, Y.S.; Patil, N.P. Factors influencing the gut microbiome in children: From infancy to childhood. J. Biosci. 2019, 44, 49.
- Zheng, Y.; Wang, Q.; Ma, L.; Chen, Y.; Gao, Y.; Zhang, G.; Cui, S.; Liang, H.; Song, L.; He, C. Shifts in the skin microbiome associated with diaper dermatitis and emollient treatment amongst infants and toddlers in China. Exp. Dermatol. 2019, 28, 1289–1297.
- Goto, T.; Yamashita, A.; Hirakawa, H.; Matsutani, M.; Todo, K.; Ohshima, K.; Toh, H.; Miyamoto, K.; Kuhara, S.; Hattori, M.; et al. Complete genome sequence of Finegoldia magna, an anaerobic opportunistic pathogen. DNA Res. 2008, 15, 39–47.
- Ferrazzini, G.; Kaiser, R.R.; Hirsig Cheng, S.-K.; Wehrli, M.; Della Casa, V.; Pohlig, G.; Gonser, S.; Graf, F.; Jörg, W. Microbiological aspects of diaper dermatitis. Dermatology 2003, 206, 136–141.
- Teufel, A.; Howard, B.; Hu, P.; Carr, A.N. Characterization of the microbiome in the infant diapered area: Insights from healthy and damaged skin. Exp. Dermatol. 2021, 30, 1409–1417.
- Bäsler, K.; Brandner, J.M. Tight junctions in skin inflammation. Pflugers Arch. Eur. J. Physiol. 2017, 469, 3–14.
- Ho, K.K.; Campbell, K.L.; Lavergne, S.N. Contact dermatitis: A comparative and translational review of the literature. Vet. Dermatol. 2015, 26, 314-e67.
- Lambers, H.; Piessens, S.; Bloem, A.; Pronk, H.; Finkel, P. Natural skin surface pH is on average below 5, which is beneficial for its resident flora. Int. J. Cosmet. Sci. 2006, 28, 359–370.
- Rippke, F.; Berardesca, E.; Weber, T.M. pH and microbial infections. Curr. Probl. Dermatol. 2018, 54, 87–94.
- Schmid-Wendtner, M.-H.; Korting, H.C. The pH of the skin surface and its impact on the barrier function. Skin Pharmacol. Physiol. 2006, 19, 296–302.
- Gosenca, M.; Gašperlin, M.; Kristl, J. Irritative contact dermatitis: From mechanism of irritation to irritants’ assessment. Farm. Vestn. 2012, 63, 145–152.
- Lee, H.Y.; Stieger, M.; Yawalkar, N.; Kakeda, M. Cytokines and chemokines in irritant contact dermatitis. Mediat. Inflamm. 2013, 2013, 916497.
- Perkins, M.A.; Osterhues, M.A.; Farage, M.A.; Robinson, M.K. A noninvasive method to assess skin irritation and compromised skin conditions using simple tape adsorption of molecular markers of inflammation. Skin Res. Technol. 2001, 7, 227–237.
- Visscher, M.O. Recent advances in diaper dermatitis: Etiology and treatment. Pediatr. Health 2009, 3, 81–98.
- Roudsari, M.R.; Karimi, R.; Sohrabvandi, S.; Mortazavian, A.M. Health effects of probiotics on the skin. Crit. Rev. Food Sci. Nutr. 2015, 55, 1219–1240.
- Cinque, B.; La Torre, C.; Melchiorre, E.; Marchesani, G.; Zoccali, G.; Palumbo, P.; Di Marzio, L.; Masci, A.; Mosca, L.; Mastromarino, P.; et al. Use of probiotics for dermal applications. Probiotics 2011, 21, 221–241.
- Saavedra, J.; Abi-Hanna, A.; Moore, N.; Yolken, R. Effect of long term consumption of infant formulas with Bifidobacteria (B) and S. thermophilus (ST) on stool patterns and diaper rash in infants. J. Pediatr. Gastroenterol. Nutr. 1998, 27, 483.
- Dimitratos, S.M.; Brown, H.; Shafizadeh, T.; Kazi, S.; Altmann, T.; Ostrer, B. Symptomatic relief from at-home use of activated Bifidobacterium infantis EVC001 probiotic in infants: Results from a consumer survey on the effects on diaper rash, colic symptoms, and sleep. Benef. Microbes 2021, 12, 333–340.
