Allografts, in turn, provide availability of material and avoid the lack of donor área
[5]. Despite the absence of viable cells, allografts maintain a native matrix that supports axonal growth, cell migration and angiogenesis
[4][10][4,10]. Although, the required immunosuppression and inherent complications are disadvantages of allografts
[3][4][7][8][3,4,7,8]. Nerve conduits are an option for the treatment of peripheral nerve injuries
[5]. Conduits are tubular structures that surround the ends of the nerve and favor the direction of axonal growth
[4]. The conduits can be constructed of synthetic materials, biodegradable or of natural components, such as veins, arteries and tendons. Nonetheless, an ideal conduit must have some fundamental properties, such as biocompatibility, biodegradability, low immunogenicity, mechanical strength and adequate permeability to allow the exchange of nutrients and biological molecules
[1]. Additionally, the success of this technique depends on the formation of a fibrin matrix in the conduit lumen, which will support vascular infiltration and cell migration
[5]. Furthermore, the use of conduits should only be indicated for the treatment of small nerve gaps (<3 cm)
[1][3][4][5][7][1,3,4,5,7].
In addition to these strategies, cell therapy emerges as an interesting alternative for treating nervous tissue injuries. Cellular components play an important role in overall tissue healing.In the same way, nerve regeneration is a complex process that requires the interaction of various types of cells, extracellular matrix components, blood vessels, cytokines and growth factors
[1]. Besides that, it is also critical that an endogenous fibrin matrix forms at the injury site in order to support axonal growth, vascular infiltration and immune system cell migration
[4][8][4,8]. Nerve regeneration process involves a sequence of steps. After the injury, morphological and molecular changes occur in nerve cells, transport of proteins and influx of ions, such as calcium
[4]. These events signal a disturbance in the homeostasis of the microenvironment that leads to changes in cell metabolism, with consequent activation of several signaling pathways and regulation of gene expression
[4]. Schwann cells, macrophages and fibroblasts act in nerve regeneration from the initial stages. Schwann cells act together with macrophages in the Wallerian degeneration process, phagocytizing fragmented axons and myelin debris
[4].
Throughout the regenerative process, the cells present in the microenvironment, mainly Schwann cells, overexpress neurotrophins and others growth factors involved with neuronal regeneration. Neurotrophins include nerve growth factor (NGF), brain derived neurotrophic factor (BDNF), neurotrophin-3 (NT-3) and neurotrophin-4/5 (NT-4/5), which are responsible for neuron growth and survival
[8][11][8,11]. Other factors stimulate cell proliferation, differentiation and survival, such as ciliary neurotrophic factor (CNTF), glial cell line-derived growth factor (GDNF) and fibroblast growth factor (FGF)
[8]. Among them, NGF is the most commonly characterized growth factor to assess the neurotrophic potential of cells
[11]. Thus, nerve regeneration process is marked by overexpression of genes related to the inflammatory response, angiogenesis, synthesis of neurotrophic factors and expression of proteins involved in axonal growth and nerve fiber myelination
[4]. Considering these issues, Schwann cells constitutes one of the most promising strategies to favor the regeneration of nervous tissue
[5][12][13][14][15][16][17][5,12,13,14,15,16,17].
However, certain limitations make it difficult to use Schwann cells, such as limited availability of donor tissue and difficulties related to the cultivation and isolation of these cells
[4][5][4,5]. As a result of these limitations, other cell lines have been investigated for use in regenerative therapy, such as embryonic stem cells (ESCs), induced pluripotent stem cells (iPSCs) and mesenchymal stem cells (MSCs)
[5][12][17][5,12,17]. Among the candidate cells, MSCs stand out as a viable alternative for use in regenerative medicine, considering the ethical and genetic manipulation issues that limit the use of ESCs and iPSCs, respectively
[12][17][12,17].
MSCs are undifferentiated cells that can be obtained from different sources, such as bone marrow, periosteum, adipose tissue, skin, muscle, tendons, umbilical cord, peripheral circulation and dental tissue
[18][19][18,19]. Due to their adherence to plastic, MSCs can be expanded in culture and characterized by the expression of specific surface antigens, such as CD29, CD73, CD90 and CD109
[17][18][20][21][22][17,18,20,21,22]. MSCs have several biological properties that favor tissue regeneration. MSCs have the capacity for self-renewal, proliferation and differentiation in different cell lines, depending on the stimulus from the microenvironment
[17][18][20][21][17,18,20,21]. Under neural induction, MSCs can differentiate into neuronal cells and express neurotrophic growth factors, such as nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF)
[22][23][24][25][26][22,23,24,25,26]. MSCs also have anti-apoptotic, angiogenic and immunomodulatory potential, and act by regulating the production of inflammatory cytokines through several signaling pathways
[19][20][21][25][27][19,20,21,25,27].
Modulation of the inflammatory response is essencial to limit tissue destruction, to reduce the formation of fibrous scars and to favor the regeneration of the injured area
[12]. Additionally, MSCs express angiogenic factors, such as vascular endothelial growth factor (VEGF), which contribute to vascular neoformation
[22][28][29][22,28,29]. MSCs are still able to migrate to the site of injury and recruit other cells through paracrine mechanisms
[21].In nervous system injuries, MSCs can be used to favor axonal regeneration and myelinization of the myelin sheath
[22][23][24][26][30][22,23,24,26,30]. Together, these characteristics make MSCs promising candidates for use in cell therapy strategies.
For use in cell therapy strategies, MSCs can be harvested from autologous tissues and implanted at the injury site through a cell delivery vehicle, such as fibrin glue or fibrin sealant. In regenerative medicine, fibrin glue can be used as a delivery system for drugs, biomolecules, growth factors and cells
[31][32][33][34][31,32,33,34]. Fibrin is a natural polymer formed by the combination of thrombin and fibrinogen, which are components of the blood coagulation system
[33][35][36][33,35,36]. Fibrin glue had its use approved by the FDA and since 1976 it has been widely used as a hemostatic agent to treat coagulopathies and in several surgical specialties, such as neurological, gastrointestinal and cardiovascular surgeries, among others
[31][35][37][31,35,37]. In medical practice, fibrin glue can be obtained from autologous blood components, which reduces the risk of immune reactions
[31][35][38][31,35,38].
Fibrin glue has several other interesting biological properties for use in regenerative therapies. Fibrin is a biocompatible matrix that can be naturally degraded by the action of fibrinolytic enzymes, such as metalloproteinases
[33][39][40][41][33,39,40,41]. When used as a cell delivery vehicle, fibrin glue has the advantage of allowing a uniform distribution of cells in the matrix and it can also be injected into the lesion area through less invasive procedures
[34][41][34,41]. Furthermore, fibrin glue provides a bioactive matrix that favors cell adhesion, viability, proliferation and differentiation
[33][34][42][33,34,42]. The fibrin glue also contains numerous binding sites with cells, molecules and growth factors that act in the tissue regeneration process
[34][41][34,41]. Thus, the fibrin glue favors cell-matrix interactions and supports axonal growth. Additionally, the porosity of the fibrin matrix favors angiogenesis and vascular infiltration, which are essential for the restoration of the injured area
[34][41][34,41].
The viability of growing MSCs in fibrin glue has been reported in several studies
[23][24][26][30][40][43][23,24,26,30,40,43]. Kalbermatten et al. (2008) reported that fibrin glue improved the adhesion of MSCs and Schwann cells in bioresorbable poly-3-hydroxybutyrate nerve conduits
[43]. This study showed that cells seeded in fibrin glue were optimally distributed along the conduit, better than those seeded in growth medium
[43]. In the study by Gardin et al. (2011), adult stem cells organized in neurospheres and seeded in fibrin glue meshes were able to grow and differentiate into glial/neuron cells under neural induction, without any chromosomal alteration
[30]. Likewise, Park et al. (2012) showed that MSCs were able to differentiate into neuronal cells, to exhibit neuron-like cell morphology and to express various neural markers and transcription factors
[23] (
Figure 1).
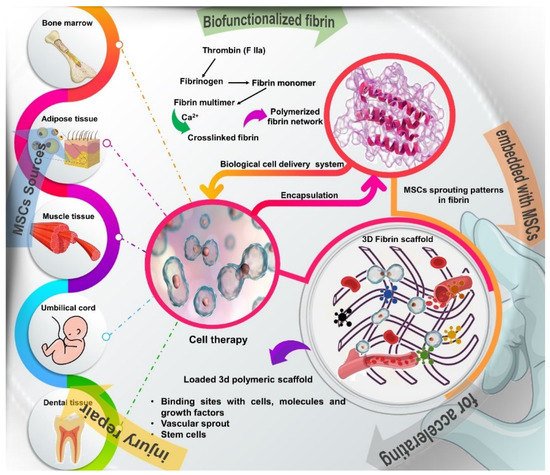
Figure 1. Schematic overview of the different sources of stem cells, such as bone marrow, adipose tissue, muscle, tendons, umbilical cord, and dental tissue and description of how to obtain the fibrin network, a natural polymer formed by the combination of thrombin and fibrinogen, which are components of the blood coagulation system. Fibrin glue provides a 3D bioactive matrix that favors cell adhesion, viability, proliferation and differentiation, in addition to containing numerous binding sites with cells, molecules and growth factors that act in the tissue regeneration process.
Further studies also demonstrated that MSCs cultivated in fibrin glue were able to differentiate into neuronal cells and to express neurogenic marker proteins, such as microtubule-associated protein 2 (MAP2), nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF) and Tau protein
[24][26][24,26].
2. Potential of Fibrin Glue and Mesenchymal Stem Cells (MSCs) to Regenerate Nerve Injuries
Studies showed that fibrin glue combined with MSCs significantly favored nerve regeneration when compared to the isolated use of fibrin glue. Therapy with MSCs favored axonal regeneration and remyelination of nerve fibers, as well as increased myelinated fiber thickness, axon number and expression of neurotrophic factors. Additionally, treatment with MSCs improved muscle weight and motor function recovery, while reducing fibrosis at the injury site. In the study by Pan et al. (2006), treatment with fibrin glue and MSCs significantly improved the compound muscle action potential (42.5 ± 1.25%) compared to the isolated use of fibrin glue (28.5 ± 1.3%)
[44][50]. Likewise, Pan et al. (2007) reported that groups treated with fibrin glue alone or associated with MSCs presented compound muscle action potential of 27.8 ± 4.22% and 67 ± 6.98%, with conduction latency of 3.91 ± 0.303 and 1.33 ± 0.048 msec, respectively
[45][48]. When used with autologous nerve graft, fibrin glue with adipose MSCs (AD-MSCs) promoted a significant increase in the number of myelinated fibers with improved motor activity and angiogenesis, compared to the use of autologous graft alone or associated with fibrin glue without cells
[46].
Likewise, the use of acellular allograft associated with fibrin glue and bone marrow MSCs (BM-MSCs) presented regenerative potential similar to the isolated use of autologous graft and superior to the use of allograft combined with fibrin glue without cells
[26]. Studies that administered agents with anti-inflammatory or immunomodulatory properties, such as Natto extract
[47][54], granulocyte colony-stimulating factor (G-CSF)
[48][53] and cyclosporine A
[49][47], concurrently with therapy with fibrin glue and MSCs, reported an additional beneficial effect on certain parameters. These studies showed a reduction in cell apoptosis, in the inflammatory response mediated by macrophages and in the levels of pro-inflammatory cytokines, such as TNF-α and IL-1β, in addition to an improvement in axonal regeneration and functional motor activity.
Nerve regeneration was also achieved in studies that enveloped the resected nerve ends with absorbable bovine collagen dura mater (Lyoplant
®)
[23][24][23,24] or fibrin conduit
[49][47] and implanted fibrin glue with MSCs into the biodegradable nerve tubule. Finally, the study that compared the effect of implantation of fibrin glue containing undifferentiated or differentiated MSCs in neuronal cells related that nerve regeneration was not affected by the cellular differentiation stage, as the two types of cells presented considerable and similar regenerative potential
[24], exhibiting greater expression of specific markers for angiogenesis, axonal fiber and myelin sheath, such as vascular endothelial growth factor receptor-1 (VEGFR-1), glial fibrillary acid protein (GFAP), S-100 protein, myelin basic protein-2 (MBP-2) and p75 nerve growth factor receptor (p75NGFR).