For hybrid electric vehicles, supercapacitors are an attractive technology which, when used in conjunction with the batteries as a hybrid system, could solve the shortcomings of the battery. Supercapacitors would allow hybrid electric vehicles to achieve high efficiency and better power control. Supercapacitors possess very good power density. Besides this, their charge-discharge cycling stability and comparatively reasonable cost make them an incredible energy-storing device. The manufacturing strategy and the major parts like electrodes, current collector, binder, separator, and electrolyte define the performance of a supercapacitor. Among these, electrode materials play an important role when it comes to the performance of supercapacitors. They resolve the charge storage in the device and thus decide the capacitance.
- supercapacitors
1. Introduction
| Comparison Parameter | Battery | Supercapacitor |
|---|---|---|
| Storage mechanism | Chemical | Physical |
| Power limitations | Reaction kinetics, mass transport | Electrolyte conductivity |
| Charge rate | Kinetically limited | High |
| Energy storage | High | Limited |
| Cycle life limitations | Mechanical stability, chemical reversibility | Side reactions |
2. Electrode Materials
Supercapacitors are divided generally into different types mainly according to the charge storage mechanism. Figure 1 shows the general classification of the supercapacitors. One is electric double-layer capacitors (EDLCs) and the other is pseudo capacitors (PCs). EDLCs are sometimes also called electrostatic capacitors. The charge storage in EDLCs takes place at the electrode/electrolyte interface through the electrostatic charge absorption mechanism. The most attractive materials for EDLCs have been carbon-based materials, mainly due to their abundance in nature and the high surface area; on the downside, the relatively low specific capacitance is a disadvantage [9][3].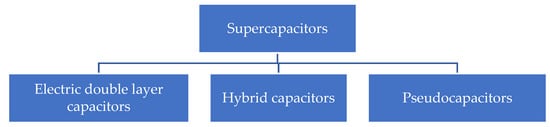
2.1. Carbonaceous Materials
Carbon-based materials are the most commonly used material for various applications in supercapacitors, thanks to their high availability and robust production processes in the industry, which in turn result in the reduced cost [15][4]. Among the applications, electrodes based on carbon materials are highly popular. They can be produced in various forms like fibers, nanotubes, and foams from 1D to the 3D structure. Usually, the electrode surface area, manufactured with carbon, is directly proportional to the specific capacitance, but this is not always the case. Some types of carbon will have higher specific capacitance even when they have a lower surface area compared to the electrode with a high specific area [16][5].2.2. Metal-Organic Framework (MOF) Based Electrode Materials
The attention metal-organic framework is receiving as a template for the synthesis of nanocomposites of porous carbon, metal/metal oxides, and porous MOs has gathered momentum recently. In general, pristine MOFs are being used as positive electrodes for supercapacitor devices, whereas MOF-derived carbon is being used as a negative one. Compared to pure carbon-based materials, these MOF-derived carbon materials deliver excellent electrochemical performance owing to their favorable natures like high porosity, high specific surface area, etc. Annealing of MOFs at high temperature under inert atmosphere converts them into carbon, retaining the original MOF template [115,116][6][7]. The preparation of MOFs has been done by combining the organic and inorganic units through solid chemical bonds.2.3. Bimetallic Metal-Organic Framework (BMOF)
To enhance the intrinsic properties of MOFs, bimetals are combined with MOF structure as they can favorably introduce high porosity and defects due to the combinational effects between the various types of metals. This enables BMOFs to be used in various applications like electrodes of supercapacitors. Producing heterojunctions with the help of bimetallic metal organic frameworks is going to provide a new way to study the effects between the mixed metal atoms and this is believed to eliminate many limitations faced during the current practical applications of electrode materials. Enhancing the performance of metal organic framework based supercapacitors by combining another metal ion into the framework has been proposed [136][8]. The electron’s electrical conductivity between electrolyte surfaces and electrodes is improved considerably as the second metal node is doped in a MOF, which could effectively endorse electronic coupling between the metal node [137][9]. Even though BMOF is manufactured from MOF, its morphology is different, but still in most of the cases it holds the crystal structure of the pristine MOF [136,137,138,139,140][8][9][10][11][12]. As the BMOF possesses improved surface area, porosity, lesser particle size, and better conductivity in comparison to MOF supercapacitors, its capacitance can be elevated. Multiple valance states of the metal species are present in BMOFs inducing higher redox sites, which makes them an interesting material in the supercapacitor electrode research studies [141][13].2.4. Conducting Polymers
Conducting polymers (CPs) enables the faradaic redox reactions and thus helps improve the specific capacitance of the supercapacitors. They are usually seen in composite materials used for the synthesis of supercapacitor electrodes. An improved pseudocapacitance is resultant due to the faradaic redox reactions. The charging of conducting polymers takes place all over the material, whereas only surface is involved for plain carbon electrodes. For the redox reaction, the ions from the electrolyte transfer into the polymer and out of it which results in an improved capacitance and also exhibits reduced cyclability [158][14]. Polyaniline (PANI) is one of the examples of this type of polymer which has undergone many studies. Its low cost, better conductivity, and comparatively easier production methods made them an attractive material.2.5. Transition Metal Oxides
Most of the research based on transition metals for supercapacitors has mainly studied the oxides of transition metals, since pseudocapacitance was discovered in 1971 [62][15]. The studies on transition metal dichalcogenides (TMDC) has begun only recently. TMDC also contains oxides like RuO2. However, as chalcogen is in the 16th group of the periodic table, transition metal dichalcogenides are not metal oxides in reality and that is why the two are labelled separately in most cases. TMDC includes oxides of metals like titanium, molybdenum, sulfides of metals like tungsten, etc. [161,162,163,164,165,166,167,168][16][17][18][19][20][21][22][23]. Figure 32 shows some of the most studied transition metal oxides.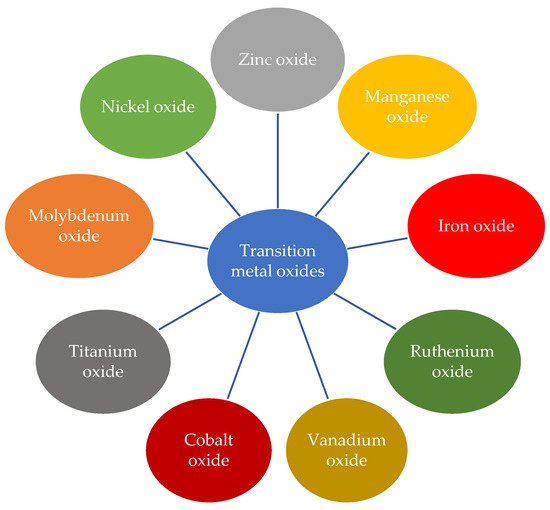
2.6. Transition Metal Nitrides
2.7. Redox Polymers
References
- González, A.; Goikolea, E.; Barrena, J.A.; Mysyk, R. Review on supercapacitors: Technologies and materials. Renew. Sustain. Energy Rev. 2016, 58, 1189–1206.
- Abdel Maksoud, M.I.A.; Fahim, R.A.; Shalan, A.E.; Abd Elkodous, M.; Olojede, S.O.; Osman, A.I.; Farrell, C.; Al-Muhtaseb, A.H.; Awed, A.S.; Ashour, A.H.; et al. Advanced Materials and Technologies for Supercapacitors Used in Energy Conversion and Storage: A Review; Springer International Publishing: Berlin/Heidelberg, Germany, 2021; Volume 19, ISBN 0123456789.
- Raj, B.; Padhy, A.K.; Basu, S.; Mohapatra, M. Review—Futuristic Direction for R&D Challenges to Develop 2D Advanced Materials Based Supercapacitors. J. Electrochem. Soc. 2020, 167, 136501.
- Xie, L.; Sun, G.; Su, F.; Guo, X.; Kong, Q.; Li, X.; Huang, X.; Wan, L.; Song, W.; Li, K.; et al. Hierarchical porous carbon microtubes derived from willow catkins for supercapacitor applications. J. Mater. Chem. A 2016, 4, 1637–1646.
- Béguin, F.; Presser, V.; Balducci, A.; Frackowiak, E. Carbons and electrolytes for advanced supercapacitors. Adv. Mater. 2014, 26, 2219–2251.
- Wang, R.; Jin, D.; Zhang, Y.; Wang, S.; Lang, J.; Yan, X.; Zhang, L. Engineering metal organic framework derived 3D nanostructures for high performance hybrid supercapacitors. J. Mater. Chem. A 2017, 5, 292–302.
- Wang, L.; Han, Y.; Feng, X.; Zhou, J.; Qi, P.; Wang, B. Metal-organic frameworks for energy storage: Batteries and supercapacitors. Coord. Chem. Rev. 2016, 307, 361–381.
- Qu, C.; Zhao, B.; Jiao, Y.; Chen, D.; Dai, S.; deglee, B.M.; Chen, Y.; Walton, K.S.; Zou, R.; Liu, M. Functionalized Bimetallic Hydroxides Derived from Metal–Organic Frameworks for High-Performance Hybrid Supercapacitor with Exceptional Cycling Stability. ACS Energy Lett. 2017, 2, 1263–1269.
- Bhardwaj, S.K.; Bhardwaj, N.; Kaur, R.; Mehta, J.; Sharma, A.L.; Kim, K.H.; Deep, A. An overview of different strategies to introduce conductivity in metal-organic frameworks and miscellaneous applications thereof. J. Mater. Chem. A 2018, 6, 14992–15009.
- Young, C.; Kim, J.; Kaneti, Y.V.; Yamauchi, Y. One-Step Synthetic Strategy of Hybrid Materials from Bimetallic Metal-Organic Frameworks for Supercapacitor Applications. ACS Appl. Energy Mater. 2018, 1, 2007–2015.
- Chen, C.; Wu, M.-K.; Tao, K.; Zhou, J.-J.; Li, Y.-L.; Han, X.; Han, L. Formation of bimetallic metal–organic framework nanosheets and their derived porous nickel–cobalt sulfides for supercapacitors. Dalt. Trans. 2018, 47, 5639–5645.
- Fu, D.; Chen, Z.; Yu, C.; Song, X.; Zhong, W. Bimetallic-organic coordination polymers to prepare N-doped hierarchical porous carbon for high performance supercapacitors. Prog. Nat. Sci. Mater. Int. 2019, 29, 495–503.
- Shinde, P.A.; Khan, M.F.; Rehman, M.A.; Jung, E.; Pham, Q.N.; Won, Y.; Jun, S.C. Nitrogen-doped carbon integrated nickel–cobalt metal phosphide marigold flowers as a high capacity electrode for hybrid supercapacitors. CrystEngComm 2020, 22, 6360–6370.
- Ates, M. Graphene and its nanocomposites used as an active materials for supercapacitors. J. Solid State Electrochem. 2016, 20, 1509–1526.
- Augustyn, V.; Simon, P.; Dunn, B. Pseudocapacitive oxide materials for high-rate electrochemical energy storage. Energy Environ. Sci. 2014, 7, 1597–1614.
- Liu, Y.; Cai, X.; Jiang, J.; Yan, M.; Shi, W. Nitrogen and carbon co-doped Ni-TiO2 spindles for high performance electrochemical capacitor electrodes. Appl. Surf. Sci. 2017, 396, 774–779.
- Jung, M.-J.; Kim, Y.; Lee, Y.-S. Enhancement of the electrochemical capacitance of TiOF2 obtained via control of the crystal structure. J. Ind. Eng. Chem. 2016, 47.
- Zhang, T.; Kong, L.B.; Liu, M.C.; Dai, Y.H.; Yan, K.; Hu, B.; Luo, Y.C.; Kang, L. Design and preparation of MoO2/MoS2 as negative electrode materials for supercapacitors. Mater. Des. 2016, 112, 88–96.
- Gigot, A.; Fontana, M.; Serrapede, M.; Castellino, M.; Bianco, S.; Armandi, M.; Bonelli, B.; Pirri, C.F.; Tresso, E.; Rivolo, P. Mixed 1T-2H Phase MoS2/Reduced Graphene Oxide as Active Electrode for Enhanced Supercapacitive Performance. ACS Appl. Mater. Interfaces 2016, 8, 32842–32852.
- Tu, C.C.; Lin, L.Y.; Xiao, B.C.; Chen, Y.S. Highly efficient supercapacitor electrode with two-dimensional tungsten disulfide and reduced graphene oxide hybrid nanosheets. J. Power Sources 2016, 320, 78–85.
- Ratha, S.; Rout, C.S. Supercapacitor electrodes based on layered tungsten disulfide-reduced graphene oxide hybrids synthesized by a facile hydrothermal method. ACS Appl. Mater. Interfaces 2013, 5, 11427–11433.
- Hu, B.; Qin, X.; Asiri, A.M.; Alamry, K.A.; Al-Youbi, A.O.; Sun, X. WS2 nanoparticles-encapsulated amorphous carbon tubes: A novel electrode material for supercapacitors with a high rate capability. Electrochem. Commun. 2013, 28, 75–78.
- Mayorga-Martinez, C.C.; Moo, J.G.S.; Khezri, B.; Song, P.; Fisher, A.C.; Sofer, Z.; Pumera, M. Self-Propelled Supercapacitors for On-Demand Circuit Configuration Based on WS2 Nanoparticles Micromachines. Adv. Funct. Mater. 2016, 26, 6662–6667.
- Alpen, U.V.; Rabenau, A.; Talat, G.H. Ionic conductivity in Li3N single crystals. Appl. Phys. Lett. 1977, 30, 621–623.
- Chen, J.G. Carbide and nitride overlayers on early transition metal surfaces: Preparation, characterization, and reactivities. Chem. Rev. 1996, 96, 1477–1498.
- Balogun, M.S.; Yu, M.; Li, C.; Zhai, T.; Liu, Y.; Lu, X.; Tong, Y. Facile synthesis of titanium nitride nanowires on carbon fabric for flexible and high-rate lithium ion batteries. J. Mater. Chem. A 2014, 2, 10825–10829.
- Gage, S.H.; Trewyn, B.G.; Ciobanu, C.V.; Pylypenko, S.; Richards, R.M. Synthetic advancements and catalytic applications of nickel nitride. Catal. Sci. Technol. 2016, 6, 4059–4076.
- Alexander, A.M.; Hargreaves, J.S.J. Alternative catalytic materials: Carbides, nitrides, phosphides and amorphous boron alloys. Chem. Soc. Rev. 2010, 39, 4388–4401.
- Liu, T.-C.; Pell, W.G.; Conway, B.E.; Roberson, S.L. Behavior of Molybdenum Nitrides as Materials for Electrochemical Capacitors: Comparison with Ruthenium Oxide. J. Electrochem. Soc. 1998, 145, 1882–1888.
- Larcher, D.; Tarascon, J. Towards greener and more sustainable batteries for electrical energy storage. Nat. Publ. Gr. 2014, 7, 19–29.
- Esser, B.; Dolhem, F.; Becuwe, M.; Poizot, P.; Vlad, A.; Brandell, D. A perspective on organic electrode materials and technologies for next generation batteries. J. Power Sources 2021, 482, 228814.
