COVID-19 is an infectious disease caused by the SARS-CoV-2 virus, responsible for an increasing number of cases and deaths. Melatonin has anti-inflammatory, antioxidant, immunomodulatory, and Mpro and MMP9 protein-inhibitory activity. Melatonin prevents SARS-CoV-2 infection, although much remains to be clarified, at high doses, it seems to have a coadjuvant therapeutic effect in the treatment of SARS-CoV-2 infection and melatonin is effective against SARS-CoV-2 infection.
- SARS-CoV-2
- COVID-19
- Melatonin
1. Introduction
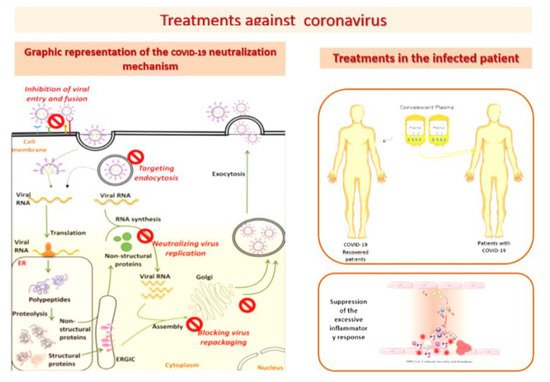
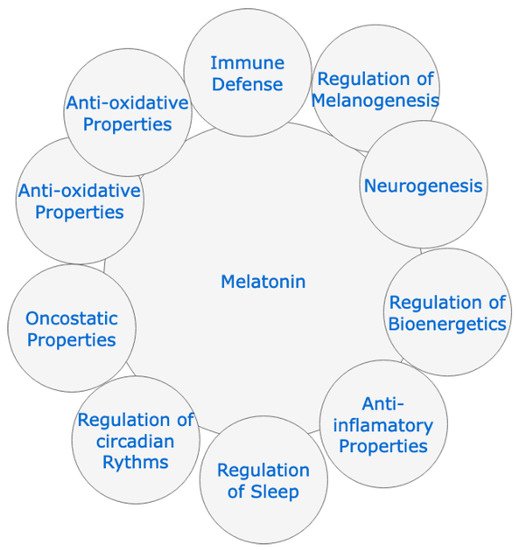
2. Protective Effect of Melatonin Administration against SARS-CoV-2 Infection
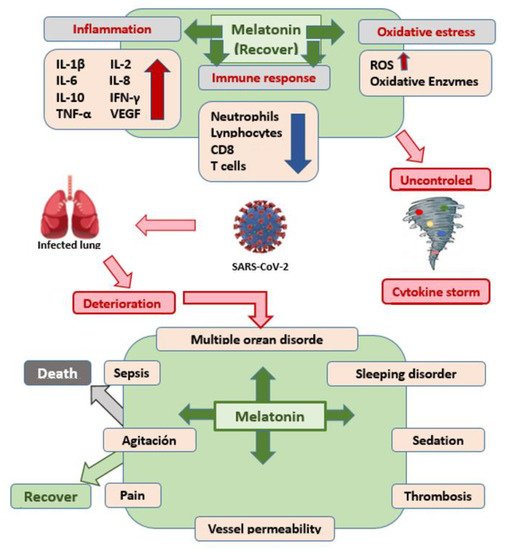
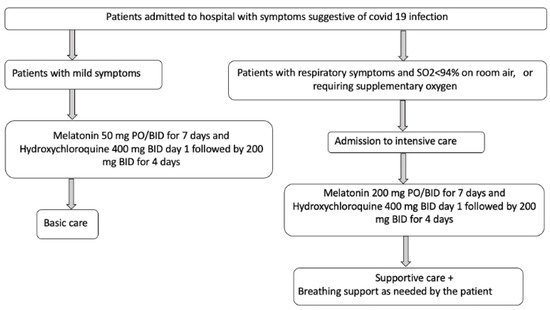
3. Conclusions
(1)Melatonin is a simple molecule, with well-documented pathophysiological functions, such as: the anti-inflammatory, antioxidant, and immunomodulatory action, as well as its inhibitory capacity of the Mpro protease and the MMP9 protein, which would make it a therapeutic alternative to consider against various infectious diseases.
(2) It has been known for years and it has been demonstrated once again with the administration of vaccines that the administration of melatonin can enhance the immune response, with a response in the rate of specific antibodies much higher than when the vaccine components are administered without melatonin.
(3) Before the onset of this pandemic, it had been shown in various viral infections that it could inhibit and/or mitigate the pathogenic action of these microbial agents in experimental animals.
(4) When melatonin is used in the laboratory (animal experimentation) and in the human clinic, a very wide safety margin has been demonstrated, well above most of the drugs used in ICUs against SARS-CoV-2 infection.
(5) We believe that there is a sufficient level of scientific evidence to authorize its use as a preventive drug against COVID-19 infection, due to its proven physiological actions, although it must be said that the exact dose to achieve this preventive effect has still not been determined.
(6) In infected patients with progressive disease, the scientific evidence is clear, and its administration is recommended for several reasons: (a) because it has been able to significantly reduce the consequences of the disease; (b) because there are no studies that say otherwise; (c) because its security profile is very broad.
(7) Although several administration guidelines have already been published in patients infected with SARS-CoV-2, it would be advisable to launch new clinical trials to define the best administration protocol, especially regarding dose and times when it should be administered, to respect its circadian rhythmicity.
References
- Jeyanathan, M.; Afkhami, S.; Smaill, F.; Miller, M.S.; Lichty, B.D.; Xing, Z. Immunological considerations for COVID-19 vaccine strategies. Nat. Rev. Immunol. 2020, 20, 615–632.
- Oran, D.P.; Topol, E.J. Prevalence of asymptomatic SARS-CoV-2 infection: A narrative review. Ann. Intern. Med. 2020, 173, 362–367.
- World Health Organization. What Happens to People Who Get Seriously Ill? Available online: https://www.who.int/news-room/q-a-detail/q-a-coronaviruses (accessed on 15 October 2020).
- Gibbons, C.L.; Mangen, M.-J.J.; Plass, D.; Havelaar, A.H.; Brooke, R.J.; Kramarz, P.; Peterson, K.L.; Stuurman, A.L.; Cassini, A.; Fèvre, E.M.; et al. Measuring underreporting and under-ascertainment in infectious disease datasets: A comparison of methods. BMC Public Health 2014, 14, 147.
- Russell, T.W.; Golding, N.; Hellewell, J.; Abbott, S.; Wright, L.; Pearson, C.A.B.; Van Zandvoort, K.; Jarvis, C.I.; Gibbs, H.; Liu, Y.; et al. Reconstructing the early global dynamics of under-ascertained COVID-19 cases and infections. BMC Med. 2020, 18, 332.
- European Centre for Disease Prevention and Control. Immune Responses and Immunity to SARS-CoV-2. Available online: https://www.ecdc.europa.eu/en/covid-19/latest-evidence/immune-responses (accessed on 15 October 2020).
- Anand, S.; Montez-Rath, M.; Han, J.; Bozeman, J.; Kerschmann, R.; Beyer, P.; Parsonnet, J.; Chertow, G.M. Prevalence of SARS-CoV-2 antibodies in a large nationwide sample of patients on dialysis in the USA: A cross-sectional study. Lancet 2020, 396, 1335–1344.
- Iwasaki, A. What reinfections mean for COVID-19. Lancet Infect. Dis. 2021, 21, 3–5.
- Liu, S.T.H.; Lin, H.-M.; Baine, I.; Wajnberg, A.; Gumprecht, J.P.; Rahman, F.; Rodriguez, D.; Tandon, P.; Bassily-Marcus, A.; Bander, J.; et al. Convalescent plasma treatment of severe COVID-19: A propensity score-matched control study. Nat. Med. 2020, 26, 1708–1713.
- Watanabe, Y.; Allen, J.D.; Wrapp, D.; McLellan, J.S.; Crispin, M. Site-specific glycan analysis of the SARS-CoV-2 spike. Science 2020, 369, 330–333.
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitsche, A.; et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020, 181, 271–280.
- Chi, X.; Yan, R.; Zhang, J.; Zhang, G.; Zhang, Y.; Hao, M.; Zhang, Z.; Fan, P.; Dong, Y.; Yang, Y.; et al. A neutralizing human antibody binds to the N-terminal domain of the Spike protein of SARS-CoV-2. Science 2020, 369, 650–655.
- Liu, L.; Wang, P.; Nair, M.S.; Yu, J.; Rapp, M.; Wang, Q.; Luo, Y.; Chan, J.F.; Sahi, V.; Figueroa, A.; et al. Potent neutralizing antibodies against multiple epitopes on SARS-CoV-2 spike. Nature 2020, 584, 450–456.
- Wrapp, D.; Wang, N.; Corbett, K.S.; Goldsmith, J.A.; Hsieh, C.-L.; Abiona, O.; Graham, B.S.; McLellan, J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 2020, 367, 1260–1263.
- Walls, A.C.; Park, Y.-J.; Tortorici, M.A.; Wall, A.; McGuire, A.T.; Veesler, D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell 2020, 181, 281–292.
- World Health Organization. Draft Landscape of COVID-19 Candidate Vaccines. Available online: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines (accessed on 29 January 2021).
- Wise, J. COVID-19: European countries suspend use of Oxford-AstraZeneca vaccine after reports of blood clots. BMJ 2021, 372, 699.
- Joint CDC and FDA Statement on Johnson & Johnson COVID-19 Vaccine. Available online: https://www.fda.gov/news-events/press-announcements/joint-cdc-and-fda-statement-johnson-johnson-covid-19-vaccine (accessed on 14 April 2021).
- Greinacher, A.; Thiele, T.; Warkentin, T.E.; Weisser, K.; Kyrle, P.A.; Eichinger, S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N. Engl. J. Med. 2021, 384, 2092–2101.
- Schultz, N.H.; Sorvoll, I.H.; Michelsen, A.E.; Munthe, L.A.; Lund-Johansen, F.; Ahlen, M.T. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N. Engl. J. Med. 2021, 384, 2124–2130.
- Connors, M.; Graham, B.S.; Lane, H.C.; Fauci, A.S. SARS-CoV-2 vaccines: Much accomplished, much to learn. Ann. Intern. Med. 2021.
- Kaur, S.P.; Gupta, V. COVID-19 vaccine: A comprehensive status report. Virus Res. 2020, 288, 198114.
- Bakhiet, M.; Taurin, S. SARS-CoV-2: Targeted managements and vaccine development. Cytokine Growth Factor Rev. 2021, 58, 16–29.
- Addetia, A.; Crawford, K.H.D.; Dingens, A.; Zhu, H.; Roychoudhury, P.; Huang, M.L. Neutralizing antibodies correlate with protection from SARS-CoV-2 in humans during a fishery vessel outbreak with a high attack rate. J. Clin. Microbiol. 2020, 58, e02107-20.
- Watanabe, Y.; Mendonça, L.; Allen, E.R.; Howe, A.; Lee, M.; Allen, J.D.; Chawla, H.; Pulido, D.; Donnellan, F.; Davies, H.; et al. Native-like SARS-CoV-2 spike glycoprotein expressed by ChAdOx1 nCoV-19/AZD1222 vaccine. ACS Cent. Sci. 2021, 7, 594–602.
- Collignon, C.; Bol, V.; Chalon, A.; Surendran, N.; Morel, S.; van den Berg, R.A. Innate immune responses to chimpanzee adenovirus vector 155 vaccination in mice and mon-keys. Front. Immunol. 2020, 11, 579872.
- Beeraka, N.M.; Sadhu, S.P.; Madhunapantula, S.V.; Pragada, R.R.; Svistunov, A.A.; Nikolenko, V.N.; Mikhaleva, L.M.; Aliev, G. Strategies for Targeting SARS-CoV-2: Small Molecule Inhibitors-The Current Status. Front. Immunol. 2020, 11, 552925.
- Anand, U.; Jakhmola, S.; Indari, O.; Jha, H.C.; Chen, Z.S.; Tripathi, V.; Pérez de la Lastra, J.M. Potential Therapeutic Targets and Vaccine Development for SARS-CoV-2/COVID-19 Pandemic Management: A Review on the Recent Update. Front. Immunol. 2021, 12, 658519.
- Anderson, G.; Reiter, R. Melatonin: Roles in influenza, COVID-19, and other viral infections. Rev. Med. Virol. 2020, 30, 1–10.
- Kleszczyński, K.; Slominski, A.T.; Steinbrink, K.; Reiter, R.J. Clinical Trials for Use of Melatonin to Fight against COVID-19 Are Urgently Needed. Nutrients 2020, 12, 2561.
- Karamitri, A.; Jockers, R. Melatonin in type 2 diabetes mellitus and obesity. Nat. Rev. Endocrinol. 2019, 15, 105–125.
- Alghamdi, B.S. The neuroprotective role of melatonin in neurological disorders. J. Neurosci. Res. 2018, 96, 1136–1149.
- Sehirli, A.O.; Sayiner, S.; Serakinci, N. Role of melatonin in the treatment of COVID-19; as an adjuvant through cluster differentiation 147 (CD147). Mol. Biol. Rep. 2020, 47, 8229–8233.
- Feitosa, E.L.; Júnior, F.T.D.S.S.; Nery Neto, J.A.O.; Matos, L.F.L.; Moura, M.H.S.; Rosales, T.O.; De Freitas, G.B.L. COVID-19: Rational discovery of the therapeutic potential of Melatonin as a SARS-CoV-2 main Protease Inhibitor. Int. J. Med. Sci. 2020, 17, 2133–2146.
- Hazra, S.; Chaudhuri, A.G.; Tiwary, B.K.; Chakrabarti, N. Matrix metallopeptidase 9 as a host protein target of chloroquine and melatonin for immunoregulation in COVID-19: A network-based meta-analysis. Life Sci. 2020, 257, 118096.
- Martín Giménez, V.M.; Prado, N.; Diez, E.; Manucha, W.; Reiter, R.J. New proposal involving nanoformulated melatonin targeted to the mitochondria as a potential COVID-19 treatment. Nanomedicine 2020, 15, 2819–2821.
- Zhang, R.; Wang, X.; Ni, L.; Di, X.; Ma, B.; Niu, S.; Liu, C.; Reiter, R.J. COVID-19Melatonin as a potential adjuvant treatment. Life Sci. 2020, 250, 117583.
- Brusco, L.; Cruz, P.; Cangas, A.; Gonzalez Rojas, C.; Vigo, D.E.; Cardinali, D.P. Efficacy of melatonin in non-intensivecare unit patients with COVID-19 pneumonia and sleep dysregulation. Melatonin Res. 2021, 4, 173–188.
- Cardinali, D.; Brown, G.; Pandi-Perumal, S.R. An urgent proposal for the immediate use of melatonin as an adjuvant to anti-SARS-CoV-2 vaccination. Melatonin Res. 2021, 4, 206–212.
- Bahrampour Juybari, K.; Pourhanifeh, M.H.; Hosseinzadeh, A.; Hemati, K.; Mehrzadi, S. Melatonin potentials against viral infections including COVID-19: Current evidence and new findings. Virus Res. 2020, 287, 198108.
- Acuña-Castroviejo, D.; Escames, G.; Figueira, J.C.; de la Oliva, P.; Borobia, A.M.; Acuña-Fernández, C. Clinical trial to test the efficacy of melatonin in COVID-19. J. Pineal. Res. 2020, 69, e12683.
- Muñoz-Hoyos, A.; Bonillo-Perales, A.; Avila-Villegas, R.; González-Ripoll, M.; Uberos, J.; Florido-Navío, J.; Molina-Carballo, A. Melatonin levels during the first week of life and their relation with the antioxidant response in the perinatal period. Neonatology 2007, 92, 209–216.
- Song, Y.; Zhang, M.; Yin, L.; Wang, K.; Zhou, Y.; Zhou, M.; Lu, Y. COVID-19 treatment: Close to a cure? A rapid review of pharmacotherapies for the novel coronavirus (SARS-CoV-2). Int. J. Antimicrob. Agents 2020, 56, 106080.
- Becker, R.C. COVID-19 treatment update: Follow the scientific evidence. J. Thromb. Thrombolysis 2020, 50, 43–53.
- Boozari, M.; Hosseinzadeh, H. Natural products for COVID-19 prevention and treatment regarding to previous coronavirus infections and novel studies. Phytoth. Res. 2021, 35, 864–876.
- Xu, X.; Han, M.; Li, T.; Sun, W.; Wang, D.; Fu, B.; Zhou, Y.; Zheng, X.; Yang, Y.; Li, X.; et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc. Natl. Acad. Sci. USA 2020, 117, 10970–10975.
- Reiter, R.J.; Abreu-González, P.; Marik, P.E.; Dominguez-Rodriguez, A. Therapeutic Algorithm for Use of Melatonin in Patients With COVID-19. Front Med. 2020, 7, 226.
