Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Lindsay Dong and Version 1 by Efrosyni Paraskeva.
Alter lipid metabolism is an emerging hallmark of cancer. The conversion of fatty acids to neutral triacylglycerides (TAG), plays a central role in this adaptive process. Acylglycerolphosphate acyltransferases (AGPATs)/lysophosphatidic acid acyltransferases (LPAATs) are a family of enzymes that catalyze the synthesis of phosphatidic acid (PA), an intermediate in TAG synthesis, a signaling molecule, and a precursor of phospholipids. Importantly, the expression of AGPATs has been linked to diverse physiological and pathological phenotypes, including cancer.
- lipids
- metabolism
- AGPAT
- phosphatidic acid
- cancer
1. The Role of Lipids in Normal and Cancer Cell Physiology
1.1. Overview of Lipid Metabolism
Lipids are a heterogeneous group of water-insoluble molecules, which includes Fatty Acids (FA), acylglycerols (e.g., tri-, di-, and mono-acylglycerides: TAG, DAG, MAG), phosphoglycerides (PG), sterols, and sphingolipids. Lipids possess several functions at cellular and organismal levels, making them critical for survival. Cellular FAs originate from exogenous uptake or de novo synthesis and are mainly used as substrates for FA oxidation and energy production or synthesis of TAGs and energy storage [1]. PGs, along with sterols and sphingolipids, are the main structural elements of biological membranes. Lipids also take part in signaling, operating as second messengers and hormones [2].
Mammalian cells acquire lipids, provided by dietary sources, the liver, or adipocytes, directly from the bloodstream as free FA or as complexes in Low- or Very-Low-Density Lipoproteins (LDL or VLDL, respectively). Lipid uptake is facilitated by specific receptors or binding proteins, such as FABPs, CD36, VLDL, and LDL receptors [3]. De novo synthesis is also an important source of lipids, especially in specific tissues such as the adipose tissue and the liver [4]. Citrate, produced in the tricarboxylic acid (TCA) cycle, is the main source of acetyl groups for FA biosynthesis. Cytoplasmic acetyl-CoA and malonyl-CoA are used to synthesize palmitate, which can be further processed (e.g., elongated, desaturated) by specific enzymes mainly in the endoplasmic reticulum (ER) (Figure 1).
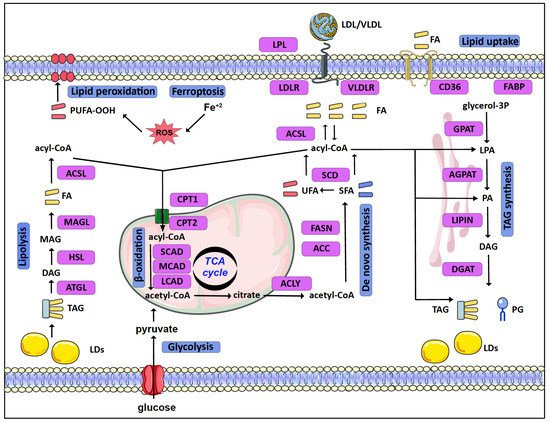
Figure 1.
Schematic overview of lipid metabolism in normal as well as cancer cells.
In eukaryotes, TAGs are mainly synthesized in the glycerol phosphate (Kennedy) pathway, in four consecutive reactions, through a stepwise addition of fatty acyl groups [5]. The pathway starts as glycerol-3-phosphate acyltransferases (GPATs) convert glycerol-3-phosphate (G3P) to lysophosphatidic acid (LPA) or monoacylglycerol-3-phosphate. In the second reaction, LPA is transformed to phosphatidic acid (PA) or diacylglycerol-3-phosphate by AGPATs. Subsequently, a family of proteins with Mg2+-dependent phosphatidic phosphatase activity, called phosphatidic acid phosphohydrolases (PAPs) or lipins (LPINs), catalyzes the dephosphorylation of PA and the formation of diacylglycerol (DAG) [6]. Thereafter, DAG is acylated by diglyceride acyltransferases (DGATs) in order to form TAG [7].
TAGs are stored in lipid droplets (LDs). LDs consist of a hydrophobic core of TAGs and sterols surrounded by a more hydrophilic phospholipid layer. LDs vary in size and number and their biogenesis and function are tightly controlled. Stored TAGs can be broken down to free FA by lipolysis. Adipose triglyceride lipase (ATGL) initiates degradation of TAG to DAG. Hormone-sensitive lipase (HSL) further hydrolyzes DAG to MAG, while MAG lipase (MAGL) hydrolyzes MAG to free FA and glycerol [5,8,9][5][8][9]. Finally, activated FAs are connected to carnitine by CPT1 (carnitine palmitoyl-transferase 1), transported to mitochondria, and broken down by β-oxidation [10].
1.2. Reprogramming of Lipid Metabolism in Cancer
Rapidly proliferating cancer cells reprogram their metabolism in order to meet increased energy and macromolecule requirements. To this end, lipidomic remodeling is a common feature of carcinogenesis (recently reviewed in [1,3,11][1][3][11]) (Figure 1). To begin with, cancer cells upregulate de novo synthesis of FA [12,13][12][13]. ATP-citrate lyase (ACLY) and acetyl-CoA carboxylase (ACC), the enzymes that convert citrate to acetyl-CoA and subsequently to malonyl-CoA in the cytoplasm, are upregulated in a number of cancers such as lung, liver, ovarian, and colorectal cancer. Likewise, FASN (fatty acid synthase) shows increased expression in cancers such as breast and prostate cancer, and a high activity of FASN has been correlated with poor disease prognosis [14,15,16][14][15][16]. These changes are mediated via the activation of lipid metabolism gene-related transcription factors, such as SREBP [17,18,19][17][18][19]. In addition to de novo synthesis, upregulation of lipid uptake by VLDL receptors and lipoprotein lipase (LPL) in breast cancer cells contributes to increased lipid uptake [21][20]. The increased FA uptake and synthesis in cancer cells can lead to an excess of free FAs and lipotoxicity. This risk is eluded by converting FA to TAGs and storing them in LDs, serving the double purpose of shielding the cell from lipotoxicity and providing fuel for energy production [9,22,23][9][21][22]. Several enzymes of the TAG synthesis and LD formation pathways are enhanced in tumors. GPAT2, a mitochondrial GPAT isoform primarily expressed in testes, was linked with breast cancer proliferation and migration [24][23], while AGPATs are also deregulated in a variety of cancers, as discussed in detail below. Interestingly, the accumulation of LDs is further increased in the hypoxic tumor microenvironment via the Hypoxia Inducible Factors (HIFs) [25][24], as part of the adaptation of cancer cells to the challenging tumor microenvironment. Accordingly, HIF-1 mediates the induction of genes involved in TAG synthesis [26,27][25][26] and LD formation [28,29,30][27][28][29]. HIF-1 further promotes lipid storage via the repression of FA oxidation [31,32][30][31]. Despite the preference of cancer cells towards lipid accumulation, the lipids stored in LDs can serve as fuels to sustain cancer cell proliferation and support tumor metastasis. To this end, upregulation of lipolysis enzymes is associated with aggressive tumor behavior [11], while β-oxidation is a significant energy source that is important for survival in many cancers [33,34][32][33]. Notably, the readjustment of lipid metabolism in cancer cells not only significantly supports the increased needs of proliferating tumor cells but can also protect them from cell death. Ferroptosis is a form of cell death that results from iron-dependent production of high amounts of reactive oxygen species (ROS) [35,36][34][35]. Lipid metabolism is directly related to the mechanism of ferroptosis via multiple ways. Specific enzymes of lipid metabolism, such as acyl-CoA synthetases (ACSL), and lipids, such as polyunsaturated fatty acids (PUFA), play important roles in the generation of lethal lipid peroxides. Highly proliferative cancer cells need higher levels of iron and membrane lipids, which make them more susceptible to ferroptosis [1,37,38][1][36][37]. Cancer cells overcome this challenge, as increased de novo synthesis of FA provides cancer cells with the flexibility to control their PUFA content via the upregulation of synthesis of saturated FAs and by storing PUFA in TAGs [1].2. The AGPAT Family
AGPATs catalyze the synthesis of PA in the second step of the TAG synthesis pathway. They are a family of five membrane-bound acyltransferases (AGPAT1-5) that specifically use acyl-CoA as acyl-donor and lysophospholipid as acyl-acceptor. The five AGPAT family isoforms are encoded by different genes. Amino acid sequence alignment of the human proteins shows that AGPAT1 and AGPAT2 share 75% of amino acid sequence homology and 35% of amino acid identity [39][38]. Likewise, AGPAT3 and AGPAT4 are evolutionarily closer to each other than to the other isoforms, sharing 75% of homology and 61% of identity [40[39][40],41], while the AGPAT5 isoform has the least overall homology with the rest of the family members, being about 38% homologous and 20% identical to AGPAT3 and AGPAT4 [42][41]. AGPAT isoforms contain four acyltransferase motifs and an HXXXXD signature, conferring catalytic activity, is present in the first acyltransferase motif of all isoforms (reviewed in [39,43][38][42]) (Figure 2).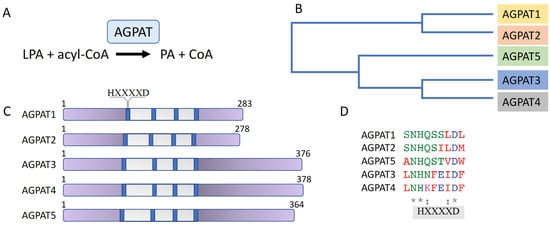
Figure 2. The AGPAT enzyme family. (A) AGPATs catalyze the conversion of LPA to PA. (B) Phylogenetic tree of AGPAT1-5. (C) Schematic representation of AGPAT isoform domain structure. The acyltransferase region is shown in grey; the four acyltransferase motifs are shown in blue. The HXXXXD signature, conferring catalytic activity, is present in the first acyltransferase motif of all isoforms. (D) Amino acid sequence alignment of the region containing the AGPAT isoforms’ HXXXXD signature. Conserved amino acids are marked by an asterisk (*).
2.1. Substrate Specificity, Membrane Localization, and Tissue Expression of the AGPAT Isoforms
This uniqueness of the AGPAT isoforms probably results from their preference for different acyl-CoA species for the production of specific PA pools. Since PA is the common precursor for phospholipids synthesis, the specificity of the different AGPATs for distinct LPA and acyl-CoA molecules defines the fatty acid composition of downstream synthesized PGs. These phospholipids, in turn, determine the structure and properties of the various biological membranes, have a role in signaling, and participate in cellular functions [39,41][38][40]. Studies on the use of acyl-CoA donors have shown that human AGPAT1 and AGPAT2 have broad acyl donor specificity for acyl-CoA [44,45,46][43][44][45]. AGPAT3 and AGPAT4, on the other hand, display a preference for polyunsaturated fatty acyl-CoA [42[41][46],47], and AGPAT3 activity has been shown to be important for the synthesis of PUFA-phospholipids in vivo [48][47].
In addition to the different substrate specificities, the distinct physiological roles of AGPATs are also connected to their subcellular localization [39][38]. AGPAT1 and AGPAT2 reside exclusively in the ER [44][43]. AGPAT3 and AGPAT4 have a broader distribution, as AGPAT3 has been localized to the ER and Golgi membranes [42,49,50][41][48][49] and AGPAT4 to the ER, Golgi, and mitochondria [47,50,51][46][49][50]. The fifth family member, AGPAT5, has only been detected on mitochondria [42,50][41][49] (Figure 3).
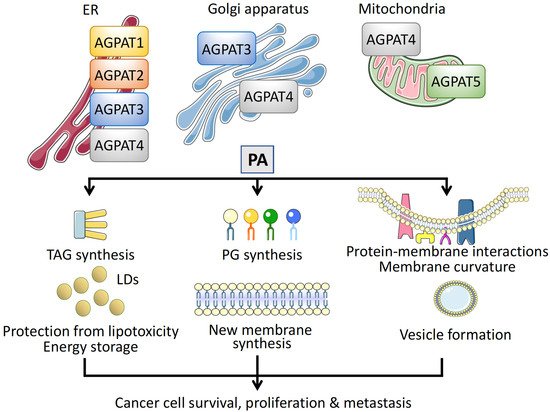

Figure 3. Intracellular localization and function of AGPATs. Different membrane compartments are populated by specific AGPAT isoforms. Isoforms AGPAT1–4 have been localized on the endoplasmic reticulum (ER), AGPAT3, and AGPAT4 on the Golgi apparatus, and AGPAT4 and AGPAT5 on mitochondria. Phosphatidic acid (PA) synthesized via the catalytic activity of AGPATs has multiple roles in cells, promoting cancer cell survival, proliferation, and metastasis. First, PA is an intermediate for the synthesis of TAGs, which are stored in lipid droplets (LDs), protecting cancer cells from free FA toxicity and serving as energy reservoirs. Second, PA is a precursor of phosphoglycerides (PGs), essential for new membrane synthesis in rapidly proliferating cancer cells. Third, insertion of PA regulates protein–membrane interactions, causes membrane curvature, and participates in vesicular transport, mediating cancer cell metastasis.
2.2. Regulation of AGPAT Expression
The multiple and distinct roles of AGPATs in physiology and disease suggest that their expression is regulated under a variety of conditions and, probably, by many transcription factors.
Nutrient availability influences the expression of AGPAT isoforms not only in the liver, the main TAG synthesis site, but also in other organs. FA supplementation increased AGPAT3 mRNA levels during muscle satellite cell differentiation [58][51]. Accordingly, in mice, acute fasting, which increases the rate of adipocyte TAG hydrolysis and FA release into the bloodstream, led to the increase of mRNA levels of AGPAT2, 3, 4, and 5 in the liver, AGPAT2 and 3 in the heart, and AGPAT1, 2, and 3 in the brain [59][52]. Models of tissue injury showed that the epidermis’ experimental barrier disruption caused a tissue-specific induction of AGPAT1, 2, 3, and 5 mRNA levels [60][53], although the transcription factors involved in both cases are not known.
Members of the PPAR family that regulate genes involved in lipid metabolism were among the first factors shown to regulate the expression of AGPATs. An early study reported that treatment with a PPARα agonist increased cardiac AGPAT activity and AGPAT3 mRNA levels of wild-type but not PPARα null mice [53][54]. The regulation of AGPAT3 expression by PPARα was further confirmed in mice with heart-specific overexpression of PPARα that had increased cardiac AGPAT3 mRNA levels, along with increased mRNAs of other enzymes involved in TAG synthesis, compared to non-transgenic animals [63][55].
HIF-1, in addition to its other target genes, which mediate the adaptation of lipid metabolism under hypoxia [25][24], has been found to also regulate the transcription of AGPAT2 gene by binding directly to the AGPAT2 promoter in human hepatocellular carcinoma and adenocarcinoma cells. AGPAT2 induction and subsequent increase of AGPAT2 protein levels were abolished upon HIF-1α silencing [27][26].
GATA-3, a transcription factor required to establish the epidermal barrier, transcriptionally regulates AGPAT5. GATA-3 binds to sites in the first intron of the AGPAT5 gene that are conserved between humans, mice, and rats; mutant GATA-3 mice had decreased expression of AGPAT5 in skin, during epidermal development [64][56].
Finally, the expression of AGPAT isoforms has been shown to be under the control of miRNAs in cancer cell lines. AGPAT1 is a predicted target of miR-122, a liver-specific miRNA, essential for the maintenance of liver homeostasis and a sensitive biomarker for liver cancer [65][57]. The miR-340-5p, which is downregulated in cisplatin-resistant osteosarcoma cell lines, targets the 3′ UTR of AGPAT2 and, although it does not affect mRNA levels, decreases the levels of AGPAT2 protein [66][58].
3. The Role of AGPATs in Cancer
3.1. PA and Cancer Cell Membrane Rearrangements
PA, the product of the AGPAT-catalyzed reaction, is the simplest glycerophospholipid in cells and acts as a precursor for the synthesis of other glycerophospholipids. PA in cells, apart from its de novo synthesis by AGPATs, is also produced by phospholipase D (PLD) and diacylglycerol kinase (DGK). However, it is not clear whether the PA subpopulation generated via each of these pathways serves different purposes within the cell [103][59].
In cancer cells, PA that is synthesized de novo by AGPATs helps to support the increased demands of membrane biogenesis. In addition, although PLD is believed to be the major producer of PA involved in the regulation of most cell signaling pathways controlling cell growth and proliferation [103][59], AGPAT2-derived PA has been shown to link the activity of mTOR with cellular lipid contents [95,104][60][61].
4.2. AGPAT Isoforms in Cancer
3.2. AGPAT Isoforms in Cancer
43.2.1. AGPAT1
In colorectal carcinoma (CRC), expression analysis of lipid metabolism genes of patient tumor samples [82][62], as well as transcriptomic meta-analysis studies of CRC patient data [83[63][64],84], showed that the increased expression of AGPAT1 is associated with a high risk of relapse and shorter survival. Accordingly, AGPAT1 has been identified as a negative prognostic marker of CRC and was included in metabolic gene signatures that could help to classify CRC patients and be used for the development of precision therapies [82,83,84][62][63][64].
On the other hand, in a microarray analysis of transcriptomic profiles for the identification of ovarian cancer mRNA biomarkers in saliva, AGPAT1 was one of the seven validated genes, with expression levels significantly downregulated in ovarian cancer patients compared to healthy controls [85][65].
43.2.2. AGPAT2
Overexpression of AGAPT2 has been detected and evaluated in several tumor types and cancer cell lines. The expression of AGPAT2 mRNA and/or protein, analyzed respectively by RT-PCR and immunohistochemistry, was increased in ovarian carcinomas compared to controls [87,88,89][66][67][68]. Furthermore, overexpression of AGPAT2 in ovarian cancer samples was associated with aggressive histology and higher tumor grade and linked to decreased overall and disease-free survival, especially in younger patients [87,89][66][68]. Therefore, AGPAT2 could be a promising prognostic tool and possibly a target of directed therapy for ovarian cancer. In osteosarcoma, AGPAT2 expression analyzed by immunohistochemistry was increased in cancer compared to adjacent tissues [91][69].
Epigenomic studies indicated that expression of AGPAT2 could potentially also be altered and, therefore, used as a biomarker in pediatric acute lymphoblastic leukemia (PALL). Specifically, the AGPAT2 gene was shown to be hypomethylated in datasets from PALL patients compared to normal blood donors in an integrative network analysis of differentially methylated (DMGs) and differentially expressed genes (DEGs) [92][70], although an earlier study did not detect any consistent differences in AGPAT2 protein levels between leukemia cell lines, cells from leukemia patients, and cells from healthy controls [111][71].
Moreover, AGPAT2 expression is necessary for survival and proliferation of many types of cancer cells and xenografts. Supporting the biological significance of the increased expression of AGPAT2 in ovarian cancer tissue samples, knockdown of AGPAT2 by siRNA decreased viability of ovarian cancer cells lines (SK-OV-3 and IGROV1) [88][67].
The profound effect of AGPAT2 expression on cancer cell proliferation suggested its possible use as a therapeutic target and led to the search and development of AGPAT2 inhibitors. A number of studies initially showed that small molecule AGAPT2 inhibitors induced apoptosis and successfully reduced cancer cell proliferation, tumor xenograft growth, and resistance to conventional chemotherapy [88,112,113,114,115,116][67][72][73][74][75][76].
43.2.3. AGPAT4
AGPAT4 was identified, together with AGPAT3, among breast cancer-expressed genes involved in endo-/exocytosis (EEC) in a reanalysis of the results from three independent genome-wide association studies [117][77]. These findings are in agreement with an analysis of transcriptome data from breast cancer patients that showed a correlation of low AGPAT4 expression with better prognosis [96][78].
In CRC patients, RNA-sequencing analysis revealed that AGPAT4 expression was increased in tumor samples compared to paracarcinoma tissues and predicted poor survival [97][79]. Interestingly, it was shown that silencing of AGPAT4 expression in a CRC cell line did not affect the growth or migration of CRC cells in vitro but suppressed CRC xenograft growth in mice. This appears to be, at least partly, mediated by the increased release of LPA from CRC cells upon AGPAT4 knockdown, which activates a systemic antitumor response via the shaping of the immune tumor microenvironment [97][79].
In addition to AGPAT4, a non-protein-coding AGPAT4 intronic transcript, lncRNA AGPAT4-IT1 was also identified to have a potential role in cancer pathogenesis. Analysis of microarray data of neuroblastoma patients identified that expression of lncRNA AGPAT4-IT1 was associated with increased patient survival [100,101][80][81].
References
- Broadfield, L.A.; Pane, A.A.; Talebi, A.; Swinnen, J.V.; Fendt, S.M. Lipid metabolism in cancer: New perspectives and emerging mechanisms. Dev. Cell 2021, 56, 1363–1393.
- Sunshine, H.; Iruela-Arispe, M.L. Membrane lipids and cell signaling. Curr. Opin. Lipidol. 2017, 28, 408–413.
- Koundouros, N.; Poulogiannis, G. Reprogramming of fatty acid metabolism in cancer. Br. J. Cancer 2020, 122, 4–22.
- Ruggles, K.V.; Turkish, A.; Sturley, S.L. Making, Baking, and Breaking: The Synthesis, Storage, and Hydrolysis of Neutral Lipids. Annu. Rev. Nutr. 2013, 33, 413–451.
- Wang, H.; Airola, M.V.; Reue, K. How lipid droplets “TAG” along: Glycerolipid synthetic enzymes and lipid storage. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2017, 1862, 1131–1145.
- Carman, G.M.; Han, G.S. Phosphatidic acid phosphatase, a key enzyme in the regulation of lipid synthesis. J. Biol. Chem. 2009, 284, 2593–2597.
- Bhatt-Wessel, B.; Jordan, T.W.; Miller, J.H.; Peng, L.F. Role of DGAT enzymes in triacylglycerol metabolism. Arch. Biochem. Biophys. 2018, 655, 1–11.
- Wang, C.W. Lipid droplets, lipophagy, and beyond. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2016, 1861, 793–805.
- Olzmann, J.A.; Carvalho, P. Dynamics and functions of lipid droplets. Nat. Rev. Mol. Cell Biol. 2019, 20, 137–155.
- Qu, Q.; Zeng, F.; Liu, X.; Wang, Q.J.; Deng, F. Fatty acid oxidation and carnitine palmitoyltransferase I: Emerging therapeutic targets in cancer. Cell Death Dis. 2016, 7, e2226.
- Currie, E.; Schulze, A.; Zechner, R.; Walther, T.C.; Farese, R.V., Jr. Cellular fatty acid metabolism and cancer. Cell Metab. 2013, 18, 153–161.
- Mashima, T.; Seimiya, H.; Tsuruo, T. De novo fatty-acid synthesis and related pathways as molecular targets for cancer therapy. Br. J. Cancer 2009, 100, 1369–1372.
- Igal, R.A. Stearoyl-CoA desaturase-1: A novel key player in the mechanisms of cell proliferation, programmed cell death and transformation to cancer. Carcinogenesis 2010, 31, 1509–1515.
- Swinnen, J.V.; Vanderhoydonc, F.; Elgamal, A.A.; Eelen, M.; Vercaeren, I.; Joniau, S.; Van Poppel, H.; Baert, L.; Goossens, K.; Heyns, W.; et al. Selective activation of the fatty acid synthesis pathway in human prostate cancer. Int. J. Cancer 2000, 88, 176–179.
- Santos, C.R.; Schulze, A. Lipid metabolism in cancer. FEBS J. 2012, 279, 2610–2623.
- Kuhajda, F.P. Fatty acid synthase and cancer: New application of an old pathway. Cancer Res. 2006, 66, 5977–5980.
- Swinnen, J.V.; Heemers, H.; Deboel, L.; Foufelle, F.; Heyns, W.; Verhoeven, G. Stimulation of tumor-associated fatty acid synthase expression by growth factor activation of the sterol regulatory element-binding protein pathway. Oncogene 2000, 19, 5173–5181.
- Li, J.N.; Mahmoud, M.A.; Han, W.F.; Ripple, M.; Pizer, E.S. Sterol regulatory element-binding protein-1 participates in the regulation of fatty acid synthase expression in colorectal neoplasia. Exp. Cell Res. 2000, 261, 159–165.
- Guo, D.; Bell, E.H.; Mischel, P.; Chakravarti, A. Targeting SREBP-1-driven lipid metabolism to treat cancer. Curr. Pharm. Des. 2014, 20, 2619–2626.
- Lupien, L.E.; Bloch, K.; Dehairs, J.; Traphagen, N.A.; Feng, W.W.; Davis, W.L.; Dennis, T.; Swinnen, J.V.; Wells, W.A.; Smits, N.C.; et al. Endocytosis of very low-density lipoproteins: An unexpected mechanism for lipid acquisition by breast cancer cells. J. Lipid Res. 2020, 61, 205–218.
- Petan, T.; Jarc, E.; Jusovic, M. Lipid Droplets in Cancer: Guardians of Fat in a Stressful World. Molecules 2018, 23, 1941.
- Cruz, A.L.S.; Barreto, E.A.; Fazolini, N.P.B.; Viola, J.P.B.; Bozza, P.T. Lipid droplets: Platforms with multiple functions in cancer hallmarks. Cell Death Dis. 2020, 11, 105.
- Pellon-Maison, M.; Montanaro, M.A.; Lacunza, E.; Garcia-Fabiani, M.B.; Soler-Gerino, M.C.; Cattaneo, E.R.; Quiroga, I.Y.; Abba, M.C.; Coleman, R.A.; Gonzalez-Baro, M.R. Glycerol-3-phosphate acyltranferase-2 behaves as a cancer testis gene and promotes growth and tumorigenicity of the breast cancer MDA-MB-231 cell line. PLoS ONE 2014, 9, e100896.
- Mylonis, I.; Simos, G.; Paraskeva, E. Hypoxia-Inducible Factors and the Regulation of Lipid Metabolism. Cells 2019, 8, 214.
- Mylonis, I.; Sembongi, H.; Befani, C.; Liakos, P.; Siniossoglou, S.; Simos, G. Hypoxia causes triglyceride accumulation by HIF-1-mediated stimulation of lipin 1 expression. J. Cell Sci. 2012, 125, 3485–3493.
- Triantafyllou, E.A.; Georgatsou, E.; Mylonis, I.; Simos, G.; Paraskeva, E. Expression of AGPAT2, an enzyme involved in the glycerophospholipid/triacylglycerol biosynthesis pathway, is directly regulated by HIF-1 and promotes survival and etoposide resistance of cancer cells under hypoxia. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2018, 1863, 1142–1152.
- Gimm, T.; Wiese, M.; Teschemacher, B.; Deggerich, A.; Schodel, J.; Knaup, K.X.; Hackenbeck, T.; Hellerbrand, C.; Amann, K.; Wiesener, M.S.; et al. Hypoxia-inducible protein 2 is a novel lipid droplet protein and a specific target gene of hypoxia-inducible factor-1. FASEB J. 2010, 24, 4443–4458.
- Maier, A.; Wu, H.; Cordasic, N.; Oefner, P.; Dietel, B.; Thiele, C.; Weidemann, A.; Eckardt, K.U.; Warnecke, C. Hypoxia-inducible protein 2 Hig2/Hilpda mediates neutral lipid accumulation in macrophages and contributes to atherosclerosis in apolipoprotein E-deficient mice. FASEB J. 2017, 31, 4971–4984.
- Zhang, X.; Saarinen, A.M.; Hitosugi, T.; Wang, Z.; Wang, L.; Ho, T.H.; Liu, J. Inhibition of intracellular lipolysis promotes human cancer cell adaptation to hypoxia. Elife 2017, 6, e31132.
- Liu, Y.; Ma, Z.; Zhao, C.; Wang, Y.; Wu, G.; Xiao, J.; McClain, C.J.; Li, X.; Feng, W. HIF-1α and HIF-2α are critically involved in hypoxia-induced lipid accumulation in hepatocytes through reducing PGC-1α-mediated fatty acid β-oxidation. Toxicol. Lett. 2014, 226, 117–123.
- Huang, D.; Li, T.; Li, X.; Zhang, L.; Sun, L.; He, X.; Zhong, X.; Jia, D.; Song, L.; Semenza, G.L.; et al. HIF-1-mediated suppression of acyl-CoA dehydrogenases and fatty acid oxidation is critical for cancer progression. Cell Rep. 2014, 8, 1930–1942.
- Camarda, R.; Zhou, A.Y.; Kohnz, R.A.; Balakrishnan, S.; Mahieu, C.; Anderton, B.; Eyob, H.; Kajimura, S.; Tward, A.; Krings, G.; et al. Inhibition of fatty acid oxidation as a therapy for MYC-overexpressing triple-negative breast cancer. Nat. Med. 2016, 22, 427–432.
- Padanad, M.S.; Konstantinidou, G.; Venkateswaran, N.; Melegari, M.; Rindhe, S.; Mitsche, M.; Yang, C.D.; Batten, K.; Huffman, K.E.; Liu, J.W.; et al. Fatty Acid Oxidation Mediated by Acyl-CoA Synthetase Long Chain 3 Is Required for Mutant KRAS Lung Tumorigenesis. Cell Rep. 2016, 16, 1614–1628.
- Hassannia, B.; Vandenabeele, P.; Vanden Berghe, T. Targeting Ferroptosis to Iron Out Cancer. Cancer Cell 2019, 35, 830–849.
- Stockwell, B.R.; Jiang, X.J.; Gu, W. Emerging Mechanisms and Disease Relevance of Ferroptosis. Trends Cell Biol. 2020, 30, 478–490.
- Li, D.; Li, Y. The interaction between ferroptosis and lipid metabolism in cancer. Signal Transduct. Target. Ther. 2020, 5, 108.
- Mou, Y.; Wang, J.; Wu, J.; He, D.; Zhang, C.; Duan, C.; Li, B. Ferroptosis, a new form of cell death: Opportunities and challenges in cancer. J. Hematol. Oncol. 2019, 12, 34.
- Bradley, R.M.; Duncan, R.E. The lysophosphatidic acid acyltransferases (acylglycerophosphate acyltransferases) family: One reaction, five enzymes, many roles. Curr. Opin. Lipidol. 2018, 29, 110–115.
- Sukumaran, S.; Barnes, R.I.; Garg, A.; Agarwal, A.K. Functional characterization of the human 1-acylglycerol-3-phosphate-O-acyltransferase isoform 10/glycerol-3-phosphate acyltransferase isoform 3. J. Mol. Endocrinol. 2009, 42, 469–478.
- Zhukovsky, M.A.; Filograna, A.; Luini, A.; Corda, D.; Valente, C. The Structure and Function of Acylglycerophosphate Acyltransferase 4/Lysophosphatidic Acid Acyltransferase Delta (AGPAT4/LPAAT delta). Front. Cell Dev. Biol. 2019, 7, 147.
- Prasad, S.S.; Garg, A.; Agarwal, A.K. Enzymatic activities of the human AGPAT isoform 3 and isoform 5: Localization of AGPAT5 to mitochondria. J. Lipid Res. 2011, 52, 451–462.
- Yamashita, A.; Hayashi, Y.; Nemoto-Sasaki, Y.; Ito, M.; Oka, S.; Tanikawa, T.; Waku, K.; Sugiura, T. Acyltransferases and transacylases that determine the fatty acid composition of glycerolipids and the metabolism of bioactive lipid mediators in mammalian cells and model organisms. Prog. Lipid Res. 2014, 53, 18–81.
- Agarwal, A.K.; Sukumaran, S.; Cortes, V.A.; Tunison, K.; Mizrachi, D.; Sankella, S.; Gerard, R.D.; Horton, J.D.; Garg, A. Human 1-acylglycerol-3-phosphate O-acyltransferase isoforms 1 and 2: Biochemical characterization and inability to rescue hepatic steatosis in Agpat2(-/-) gene lipodystrophic mice. J. Biol. Chem. 2011, 286, 37676–37691.
- Yamashita, A.; Nakanishi, H.; Suzuki, H.; Kamata, R.; Tanaka, K.; Waku, K.; Sugiura, T. Topology of acyltransferase motifs and substrate specificity and accessibility in 1-acyl-sn-glycero-3-phosphate acyltransferase 1. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2007, 1771, 1202–1215.
- Hollenback, D.; Bonham, L.; Law, L.; Rossnagle, E.; Romero, L.; Carew, H.; Tompkins, C.K.; Leung, D.W.; Singer, J.W.; White, T. Substrate specificity of lysophosphatidic acid acyltransferase beta—Evidence from membrane and whole cell assays. J. Lipid Res. 2006, 47, 593–604.
- Eto, M.; Shindou, H.; Shimizu, T. A novel lysophosphatidic acid acyltransferase enzyme (LPAAT4) with a possible role for incorporating docosahexaenoic acid into brain glycerophospholipids. Biochem. Biophys. Res. Commun. 2014, 443, 718–724.
- Hishikawa, D.; Yanagida, K.; Nagata, K.; Kanatani, A.; Iizuka, Y.; Hamano, F.; Yasuda, M.; Okamura, T.; Shindou, H.; Shimizu, T. Hepatic Levels of DHA-Containing Phospholipids Instruct SREBP1-Mediated Synthesis and Systemic Delivery of Polyunsaturated Fatty Acids. Iscience 2020, 23, 101495.
- Schmidt, J.A.; Brown, W.J. Lysophosphatidic acid acyltransferase 3 regulates Golgi complex structure and function. J. Cell Biol. 2009, 186, 211–218.
- Pagliuso, A.; Valente, C.; Giordano, L.L.; Filograna, A.; Li, G.L.; Circolo, D.; Turacchio, G.; Marzullo, V.M.; Mandrich, L.; Zhukovsky, M.A.; et al. Golgi membrane fission requires the CtBP1-S/BARS-induced activation of lysophosphatidic acid acyltransferase delta. Nat. Commun. 2016, 7, 1–15.
- Bradley, R.M.; Marvyn, P.M.; Aristizabal Henao, J.J.; Mardian, E.B.; George, S.; Aucoin, M.G.; Stark, K.D.; Duncan, R.E. Acylglycerophosphate acyltransferase 4 (AGPAT4) is a mitochondrial lysophosphatidic acid acyltransferase that regulates brain phosphatidylcholine, phosphatidylethanolamine, and phosphatidylinositol levels. Biochim. Biophys. Acta 2015, 1851, 1566–1576.
- Valentine, W.J.; Tokuoka, S.M.; Hishikawa, D.; Kita, Y.; Shindou, H.; Shimizu, T. LPAAT3 incorporates docosahexaenoic acid into skeletal muscle cell membranes and is upregulated by PPAR delta activation. J. Lipid Res. 2018, 59, 184–194.
- Bradley, R.M.; Mardian, E.B.; Moes, K.A.; Duncan, R.E. Acute Fasting Induces Expression of Acylglycerophosphate Acyltransferase (AGPAT) Enzymes in Murine Liver, Heart, and Brain. Lipids 2017, 52, 457–461.
- Lu, B.; Jiang, Y.J.; Man, M.Q.; Brown, B.; Elias, P.M.; Feingold, K.R. Expression and regulation of 1-acyl-sn-glycerol-3-phosphate acyltransferases in the epidermis. J. Lipid Res. 2005, 46, 2448–2457.
- Lu, B.; Jiang, Y.J.; Zhou, Y.L.; Xu, F.Y.; Hatch, G.M.; Choy, P.C. Cloning and characterization of murine 1-acyl-sn-glycerol 3-phosphate acyltransferases and their regulation by PPAR alpha in murine heart. Biochem. J. 2005, 385, 469–477.
- Banke, N.H.; Wende, A.R.; Leone, T.C.; O’Donnell, J.M.; Abel, E.D.; Kelly, D.P.; Lewandowski, E.D. Preferential oxidation of triacylglyceride-derived fatty acids in heart is augmented by the nuclear receptor PPARalpha. Circ. Res. 2010, 107, 233–241.
- de Guzman Strong, C.; Wertz, P.W.; Wang, C.; Yang, F.; Meltzer, P.S.; Andl, T.; Millar, S.E.; Ho, I.C.; Pai, S.Y.; Segre, J.A. Lipid defect underlies selective skin barrier impairment of an epidermal-specific deletion of Gata-3. J. Cell Biol. 2006, 175, 661–670.
- Thakral, S.; Ghoshal, K. miR-122 is a Unique Molecule with Great Potential in Diagnosis, Prognosis of Liver Disease, and Therapy Both as miRNA Mimic and Antimir. Curr. Gene Ther. 2015, 15, 142–150.
- Song, L.; Duan, P.; Gan, Y.; Li, P.; Zhao, C.; Xu, J.; Zhang, Z.; Zhou, Q. MicroRNA-340-5p modulates cisplatin resistance by targeting LPAAT beta in osteosarcoma. Braz. J. Med. Biol. Res. 2017, 50, 6359.
- Foster, D.A.; Salloum, D.; Menon, D.; Frias, M.A. Phospholipase D and the maintenance of phosphatidic acid levels for regulation of mammalian target of rapamycin (mTOR). J. Biol. Chem. 2014, 289, 22583–22588.
- Blaskovich, M.A.; Yendluri, V.; Lawrence, H.R.; Lawrence, N.J.; Sebti, S.M.; Springett, G.M. Lysophosphatidic acid acyltransferase beta regulates mTOR signaling. PLoS ONE 2013, 8, e78632.
- Zhang, C.B.; Wendel, A.A.; Keogh, M.R.; Harris, T.E.; Chen, J.; Coleman, R.A. Glycerolipid signals alter mTOR complex 2 (mTORC2) to diminish insulin signaling. Proc. Natl. Acad. Sci. USA 2012, 109, 1667–1672.
- Vargas, T.; Moreno-Rubio, J.; Herranz, J.; Cejas, P.; Molina, S.; Gonzalez-Vallinas, M.; Mendiola, M.; Burgos, E.; Aguayo, C.; Custodio, A.B.; et al. ColoLipidGene: Signature of lipid metabolism-related genes to predict prognosis in stage-II colon cancer patients. Oncotarget 2015, 6, 7348–7363.
- Fernandez, L.P.; Ramos-Ruiz, R.; Herranz, J.; Martin-Hernandez, R.; Vargas, T.; Mendiola, M.; Guerra, L.; Reglero, G.; Feliu, J.; Ramirez de Molina, A. The transcriptional and mutational landscapes of lipid metabolism-related genes in colon cancer. Oncotarget 2018, 9, 5919–5930.
- Ren, J.; Feng, J.; Song, W.; Wang, C.; Ge, Y.; Fu, T. Development and validation of a metabolic gene signature for predicting overall survival in patients with colon cancer. Clin. Exp. Med. 2020, 20, 535–544.
- Lee, Y.H.; Kim, J.H.; Zhou, H.; Kim, B.W.; Wong, D.T. Salivary transcriptomic biomarkers for detection of ovarian cancer: For serous papillary adenocarcinoma. J. Mol. Med. 2012, 90, 427–434.
- Niesporek, S.; Denkert, C.; Weichert, W.; Kobel, M.K.; Noske, A.; Sehouli, J.; Singer, J.W.; Dietel, M.; Hauptmann, S. Expression of lysophosphatidic acid acyltransferase beta (LPAAT-beta) in ovarian carcinoma: Correlation with tumour grading and prognosis. Br. J. Cancer 2005, 92, 1729–1736.
- Springett, G.M.; Bonham, L.; Hummer, A.; Linkov, I.; Misra, D.; Ma, C.; Pezzoni, G.; Di Giovine, S.; Singer, J.; Kawasaki, H.; et al. Lysophosphatidic acid acyltransferase-beta is a prognostic marker and therapeutic target in gynecologic malignancies. Cancer Res. 2005, 65, 9415–9425.
- Diefenbach, C.S.; Soslow, R.A.; Iasonos, A.; Linkov, I.; Hedvat, C.; Bonham, L.; Singer, J.; Barakat, R.R.; Aghajanian, C.; Dupont, J. Lysophosphatidic acid acyltransferase-beta (LPAAT-beta) is highly expressed in advanced ovarian cancer and is associated with aggressive histology and poor survival. Cancer 2006, 107, 1511–1519.
- Song, L.; Yang, J.; Duan, P.; Xu, J.Z.; Luo, X.D.; Luo, F.; Zhang, Z.H.; Hu, T.Y.; Liu, B.; Zhou, Q. MicroRNA-24 inhibits osteosarcoma cell proliferation both in vitro and in vivo by targeting LPAAT beta. Arch. Biochem. Biophys. 2013, 535, 128–135.
- Sanchez, R.; Mackenzie, S.A. Integrative Network Analysis of Differentially Methylated and Expressed Genes for Biomarker Identification in Leukemia. Sci. Rep. 2020, 10, 1–12.
- Douvas, M.G.; Hogan, K.N.; Ji, Y.S.; Hollenback, D.; Bonham, L.; Singer, J.W.; Mitchell, B.S. Effect of lysophosphatidic acid acyltransferase-beta inhibition in acute leukemia. Leuk. Res. 2006, 30, 1027–1036.
- Hideshima, T.; Chauhan, D.; Hayashi, T.; Podar, K.; Akiyama, M.; Mitsiades, C.; MItsiades, N.; Gong, B.Q.; Bonham, L.; de Vries, P.; et al. Antitumor activity of lysophosphatidic acid acyltransferase-beta inhibitors, a novel class of agents, in multiple myeloma. Cancer Res. 2003, 63, 8428–8436.
- Hideshima, T.; Chauhan, D.; Ishitsuka, K.; Yasui, H.; Raje, N.; Kumar, S.; Podar, K.; Mitsiades, C.; Hideshima, H.; Bonham, L.; et al. Molecular characterization of PS-341 (bortezomib) resistance: Implications for overcoming resistance using lysophosphatidic acid acyltransferase (LPAAT)-beta inhibitors. Oncogene 2005, 24, 3121–3129.
- Coon, M.; Ball, A.; Pound, J.; Ap, S.; Hollenback, D.; White, T.; Tulinsky, J.; Bonham, L.; Morrison, D.K.; Finney, R.; et al. Inhibition of lysophosphatidic acid acyltransferase beta disrupts proliferative and survival signals in normal cells and induces apoptosis of tumor cells. Mol. Cancer Ther. 2003, 2, 1067–1078.
- La Rosee, P.; Jia, T.P.; Demehri, S.; Hartel, N.; de Vries, P.; Bonham, L.; Hollenback, D.; Singer, J.W.; Melo, J.V.; Druker, B.J.; et al. Antileukemic activity of lysophosphatidic acid acyltransferase-beta inhibitor CT32228 in chronic myelogenous leukemia sensitive and resistant to imatinib. Clin. Cancer Res. 2006, 12, 6540–6546.
- Pagel, J.M.; Laugen, C.; Bonham, L.; Hackman, R.C.; Hockenbery, D.M.; Bhatt, R.; Hollenback, D.; Carew, H.; Singer, J.W.; Press, O.W. Induction of apoptosis using inhibitors of lysophosphatidic acid acyltransferase-beta and anti-CD20 monoclonal antibodies for treatment of human non-Hodgkin’s lymphomas. Clin. Cancer Res. 2005, 11, 4857–4866.
- Wittkowski, K.M.; Dadurian, C.; Seybold, M.P.; Kim, H.S.; Hoshino, A.; Lyden, D. Complex polymorphisms in endocytosis genes suggest alpha-cyclodextrin as a treatment for breast cancer. PLoS ONE 2018, 13, e0199012.
- Doria, M.L.; Ribeiro, A.S.; Wang, J.; Cotrim, C.Z.; Domingues, P.; Williams, C.; Domingues, M.R.; Helguero, L.A. Fatty acid and phospholipid biosynthetic pathways are regulated throughout mammary epithelial cell differentiation and correlate to breast cancer survival. FASEB J. 2014, 28, 4247–4264.
- Zhang, D.P.; Shi, R.C.; Xiang, W.; Kang, X.; Tang, B.; Li, C.; Gao, L.F.; Zhang, X.; Zhang, L.L.; Dai, R.Y.; et al. The Agpat4/LPA axis in colorectal cancer cells regulates antitumor responses via p38/p65 signaling in macrophages. Signal Transduct. Target. Ther. 2020, 5, 1–13.
- Meng, X.Y.; Fang, E.H.; Zhao, X.; Feng, J.X. Identification of prognostic long noncoding RNAs associated with spontaneous regression of neuroblastoma. Cancer Med. 2020, 9, 3800–3815.
- Zhong, X.D.; Tao, Y.; Chang, J.; Zhang, Y.T.; Zhang, H.; Wang, L.Y.; Liu, Y.N. Prognostic Signature of Immune Genes and Immune-Related LncRNAs in Neuroblastoma: A Study Based on GEO and TARGET Datasets. Front. Oncol. 2021, 11, 452.
More
