Lipid droplets, classically regarded as static storage organelles, are currently considered as dynamic structures involved in key processes of lipid metabolism, cellular homeostasis and signaling. Studies on the inflammatory state of atherosclerotic plaques suggest that circulating monocytes interact with products released by endothelial cells and may acquire a foamy phenotype before crossing the endothelial barrier and differentiating into macrophages. One such compound released in significant amounts into the bloodstream is arachidonic acid, the common precursor of eicosanoids, and a potent inducer of neutral lipid synthesis and lipid droplet formation in circulating monocytes. Members of the family of phospholipase A2, which hydrolyze the fatty acid present at the sn-2 position of phospholipids, have recently emerged as key controllers of lipid droplet homeostasis, regulating their formation and the availability of fatty acids for lipid mediator production.
- lipid droplet
- phospholipase A2
- arachidonic acid
- atherosclerosis
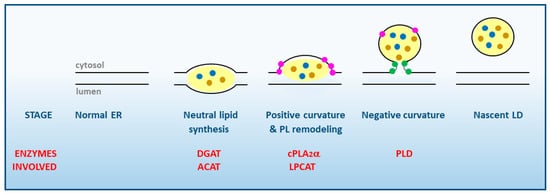
1. Lipid Droplet Biogenesis. General Aspects
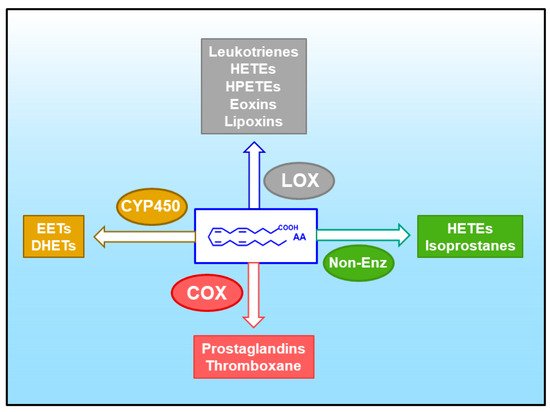
2. Arachidonic Acid, a Compound Released in Atherosclerotic Lesions
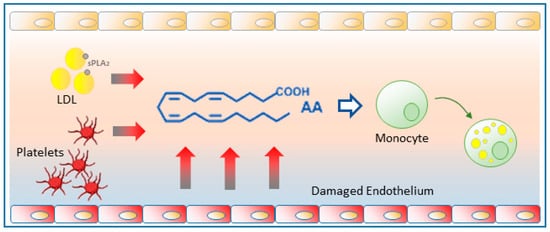
3. Glycerophospholipid Hydrolysis as a Major Pathway for the Mobilization of AA
References
- Olzmann, J.A.; Carvalho, P. Dynamics and Functions of Lipid Droplets. Nat. Rev. Mol. Cell Biol. 2019, 20, 137–155.
- Grillitsch, K.; Connerth, M.; Köfeler, H.; Arrey, T.N.; Rietschel, B.; Wagner, B.; Karas, M.; Daum, G. Lipid Particles/Droplets of the Yeast Saccharomyces Cerevisiae Revisited: Lipidome Meets Proteome. Biochim. Biophys. Acta 2011, 1811, 1165–1176.
- Thiam, A.R.; Farese, R.V., Jr.; Walther, T.C. The Biophysics and Cell Biology of Lipid Droplets. Nat. Rev. Mol. Cell Biol. 2013, 14, 775–786.
- Brasaemle, D.L. The Perilipin Family of Structural Lipid Droplet Proteins: Stabilization of Lipid Droplets and Control of Lipolysis. J. Lipid Res. 2007, 48, 2547–2559.
- Sun, Z.; Gong, J.; Wu, H.; Xu, W.; Wu, L.; Xu, D.; Gao, J.; Wu, J.W.; Yang, H.; Yang, M.; et al. Perilipin1 Promotes Unilocular Lipid Droplet Formation through the Activation of Fsp27 in Adipocytes. Nat. Commun. 2013, 4, 1594.
- Mardani, I.; Dalen, K.I.; Drevinge, C.; Miljanovic, A.; Ståhlman, M.; Klevstig, M.; Täng, M.S.; Fogelstrand, P.; Levin, M.; Ekstrand, M.; et al. Plin2-Deficiency Reduces Lipophagy and Results in Increased Lipid Accumulation in the Heart. Sci. Rep. 2019, 9, 6909.
- MacPherson, R.E.K.; Vandenboom, R.; Roy, B.D.; Peters, S.J. Skeletal Muscle PLIN3 and PLIN5 Are Serine Phosphorylated at Rest and Following Lipolysis during Adrenergic or Contractile Stimulation. Physiol. Rep. 2013, 1, e00084.
- Čopič, A.; Antoine-Bally, S.; Giménez-Andrés, M.; La Torre Garay, C.; Antonny, B.; Manni, M.M.; Pagnotta, S.; Guihot, J.; Jackson, C.L. A Giant Amphipathic Helix from a Perilipin That Is Adapted for Coating Lipid Droplets. Nat. Commun. 2018, 9, 1332.
- Sanchez, P.B.M.; Krizanac, M.; Weiskirchen, R.; Asimakopoulos, A. Understanding the Role of Perilipin 5 in Non-Alcoholic Fatty Liver Disease and Its Role in Hepatocellular Carcinoma: A Review of Novel Insights. Int. J. Mol. Sci. 2021, 22, 5284.
- Lass, A.; Zimmermann, R.; Oberer, M.; Zechner, R. Lipolysis-A Highly Regulated Multi-Enzyme Complex Mediates the Catabolism of Cellular Fat Stores. Prog. Lipid Res. 2011, 50, 14–27.
- Shen, W.J.; Patel, S.; Miyoshi, H.; Greenberg, A.S.; Kraemer, F.B. Functional Interaction of Hormone-Sensitive Lipase and Perilipin in Lipolysis. J. Lipid Res. 2009, 50, 2306–2313.
- Krahmer, N.; Guo, Y.; Wilfling, F.; Hilger, M.; Lingrell, S.; Heger, K.; Newman, H.W.; Schmidt-Supprian, M.; Vance, D.E.; Mann, M.; et al. Phosphatidylcholine Synthesis for Lipid Droplet Expansion Is Mediated by Localized Activation of CTP:Phosphocholine Cytidylyltransferase. Cell Metab. 2011, 14, 504–515.
- Aitchison, A.J.; Arsenault, D.J.; Ridgway, N.D. Nuclear-Localized CTP: Phosphocholine Cytidylyltransferase α Regulates Phosphatidylcholine Synthesis Required for Lipid Droplet Biogenesis. Mol. Biol. Cell 2015, 26, 2927–2938.
- Moessinger, C.; Kuerschner, L.; Spandl, J.; Shevchenko, A.; Thiele, C. Human Lysophosphatidylcholine Acyltransferases 1 and 2 Are Located in Lipid Droplets Where They Catalyze the Formation of Phosphatidylcholine. J. Biol. Chem. 2011, 286, 21330–21339.
- Poppelreuther, M.; Rudolph, B.; Du, C.; Grossmann, R.; Becker, M.; Thiele, C.; Ehehalt, R.; Füllekrug, J. The N-Terminal Region of Acyl-CoA Synthetase 3 Is Essential for Both the Localization on Lipid Droplets and the Function in Fatty Acid Uptake. J. Lipid Res. 2012, 53, 888–900.
- Valdearcos, M.; Esquinas, E.; Meana, C.; Gil-de-Gómez, L.; Guijas, C.; Balsinde, J.; Balboa, M.A. Subcellular Localization and Role of Lipin-1 in Human Macrophages. J. Immunol. 2011, 186, 6004–6013.
- Sembongi, H.; Miranda, M.; Han, G.S.; Fakas, S.; Grimsey, N.; Vendrell, J.; Carman, G.M.; Siniossoglou, S. Distinct Roles of the Phosphatidate Phosphatases Lipin 1 and 2 during Adipogenesis and Lipid Droplet Biogenesis in 3T3-L1 Cells. J. Biol. Chem. 2013, 288, 34502–34513.
- Melo, R.C.N.; Weller, P.F. Unraveling the Complexity of Lipid Body Organelles in Human Eosinophils. J. Leukoc. Biol. 2014, 96, 703–712.
- Yu, W.; Bozza, P.T.; Tzizik, D.M.; Gray, J.P.; Cassara, J.; Dvorak, A.M.; Weller, P.F. Co-Compartmentalization of MAP Kinases and Cytosolic Phospholipase A2 at Cytoplasmic Arachidonate-Rich Lipid Bodies. Am. J. Pathol. 1998, 152, 759–769.
- Wooten, R.E.; Willingham, M.C.; Daniel, L.W.; Leslie, C.C.; Rogers, L.C.; Sergeant, S.; O’Flaherty, J.T. Novel Translocation Responses of Cytosolic Phospholipase A2α Fluorescent Proteins. Biochim. Biophys. Acta 2008, 1783, 1544–1550.
- Jarc, E.; Petan, T. A Twist of FATe: Lipid Droplets and Inflammatory Lipid Mediators. Biochimie 2020, 169, 69–87.
- Pérez-Chacón, G.; Astudillo, A.M.; Ruipérez, V.; Balboa, M.A.; Balsinde, J. Signaling Role for Lysophosphatidylcholine Acyltransferase 3 in Receptor-Regulated Arachidonic Acid Reacylation Reactions in Human Monocytes. J. Immunol. 2010, 184, 1071–1078.
- Arrese, E.L.; Saudale, F.Z.; Soulages, J.L. Lipid Droplets as Signaling Platforms Linking Metabolic and Cellular Functions. Lipid Insights 2014, 7, 7–16.
- Guijas, C.; Rodríguez, J.P.; Rubio, J.M.; Balboa, M.A.; Balsinde, J. Phospholipase A2 Regulation of Lipid Droplet Formation. Biochim. Biophys. Acta 2014, 1841, 1661–1671.
- Wilfling, F.; Wang, H.; Haas, J.T.; Krahmer, N.; Gould, T.J.; Uchida, A.; Cheng, J.X.; Graham, M.; Christiano, R.; Fröhlich, F.; et al. Triacylglycerol Synthesis Enzymes Mediate Lipid Droplet Growth by Relocalizing from the ER to Lipid Droplets. Dev. Cell 2013, 24, 384–399.
- Jackson, C.L. Lipid Droplet Biogenesis. Curr. Opin. Cell Biol. 2018, 59, 88–96.
- Coleman, R.A.; Lee, D.P. Enzymes of Triacylglycerol Synthesis and Their Regulation. Prog. Lipid Res. 2004, 43, 134–176.
- Thiam, A.R.; Ikonen, E. Lipid Droplet Nucleation. Trends Cell Biol. 2021, 31, 108–118.
- Pol, A.; Gross, S.P.; Parton, R.G. Biogenesis of the Multifunctional Lipid Droplet: Lipids, Proteins, and Sites. J. Cell Biol. 2014, 204, 635–646.
- Guijas, C.; Pérez-Chacón, G.; Astudillo, A.M.; Rubio, J.M.; Gil-de-Gómez, L.; Balboa, M.A.; Balsinde, J. Simultaneous Activation of p38 and JNK by Arachidonic Acid Stimulates the Cytosolic Phospholipase A2-Dependent Synthesis of Lipid Droplets in Human Monocytes. J. Lipid Res. 2012, 53, 2343–2354.
- Gubern, A.; Casas, J.; Barceló-Torns, M.; Barneda, D.; de la Rosa, X.; Masgrau, R.; Picatoste, F.; Balsinde, J.; Balboa, M.A.; Claro, E. Group IVA Phospholipase A2 Is Necessary for the Biogenesis of Lipid Droplets. J. Biol. Chem. 2008, 283, 27369–27382.
- Gubern, A.; Barceló-Torns, M.; Casas, J.; Barneda, D.; Masgrau, R.; Picatoste, F.; Balsinde, J.; Balboa, M.A.; Claro, E. Lipid Droplet Biogenesis Induced by Stress Involves Triacylglycerol Synthesis that Depends on Group VIA Phospholipase A2. J. Biol. Chem. 2009, 284, 5697–5708.
- Gubern, A.; Barceló-Torns, M.; Barneda, D.; López, J.M.; Masgrau, R.; Picatoste, F.; Chalfant, C.E.; Balsinde, J.; Balboa, M.A.; Claro, E. JNK and Ceramide Kinase Govern the Biogenesis of Lipid Droplets through Activation of Group IVA Phospholipase A2. J. Biol. Chem. 2009, 284, 32359–32369.
- Leslie, C.C. Cytosolic Phospholipase A2: Physiological Function and Role in Disease. J. Lipid Res. 2015, 56, 1386–1402.
- Balsinde, J.; Winstead, M.V.; Dennis, E.A. Phospholipase A2 Regulation of Arachidonic Acid Mobilization. FEBS Lett. 2002, 531, 2–6.
- Dennis, E.A.; Norris, P.C. Eicosanoid Storm in Infection and Inflammation. Nat. Rev. Immunol. 2015, 15, 511–523.
- Astudillo, A.M.; Balboa, M.A.; Balsinde, J. Selectivity of Phospholipid Hydrolysis by Phospholipase A2 Enzymes in Activated Cells Leading to Polyunsaturated Fatty Acid Mobilization. Biochim. Biophys. Acta 2019, 1864, 772–783.
- Melo, R.C.N.; Weller, P. Lipid Droplets in Leukocytes: Organelles Linked to Inflammatory Responses. Exp. Cell. Res. 2016, 340, 193–197.
- Bozza, P.T.; Bakker-Abreu, I.; Navarro-Xavier, R.A.; Bandeira-Melo, C. Lipid Body Function in Eicosanoid Synthesis: An Update. Prostaglandins Leukot. Essent. Fatty Acids 2011, 85, 205–213.
- Kooijman, E.E.; Chupin, V.; Fuller, N.L.; Kozlov, M.M.; de Kruijff, B.; Burger, K.N.; Rand, P.R. Spontaneous Curvature of Phosphatidic Acid and Lysophosphatidic Acid. Biochemistry 2005, 44, 2097–2102.
- Andersson, L.; Boström, P.; Ericson, J.; Rutberg, M.; Magnusson, B.; Marchesan, D.; Ruiz, M.; Asp, L.; Huang, P.; Frohman, M.A.; et al. PLD1 and ERK2 Regulate Cytosolic Lipid Droplet Formation. J. Cell Sci. 2006, 119, 2246–2257.
- Balsinde, J.; Diez, E.; Fernández, B.; Mollinedo, F. Biochemical Characterization of Phospholipase D Activity from Human Neutrophils. Eur. J. Biochem. 1989, 186, 717–724.
- Tan, J.S.; Seow, C.J.; Goh, V.J.; Silver, D.L. Recent Advances in Understanding Proteins Involved in Lipid Droplet Formation, Growth and Fusion. J. Genet. Genom. 2014, 41, 251–259.
- Sprecher, H. Metabolism of Highly Unsaturated N-3 and N-6 Fatty Acids. Biochim. Biophys. Acta 2000, 1486, 219–231.
- Pérez-Chacón, G.; Astudillo, A.M.; Balgoma, D.; Balboa, M.A.; Balsinde, J. Control of Free Arachidonic Acid Levels by Phospholipases A2 and Lysophospholipid Acyltransferases. Biochim. Biophys. Acta 2009, 1791, 1103–1113.
- Astudillo, A.M.; Balgoma, D.; Balboa, M.A.; Balsinde, J. Dynamics of Arachidonic Acid Mobilization by Inflammatory Cells. Biochim. Biophys. Acta 2012, 1821, 249–256.
- Guijas, C.; Astudillo, A.M.; Gil-de-Gómez, L.; Rubio, J.M.; Balboa, M.A.; Balsinde, J. Phospholipid Sources for Adrenic Acid Mobilization in RAW 264.7 Macrophages: Comparison with Arachidonic Acid. Biochim. Biophys. Acta 2012, 1821, 1386–1393.
- Monge, P.; Garrido, A.; Rubio, J.M.; Magrioti, V.; Kokotos, G.; Balboa, M.A.; Balsinde, J. The Contribution of Cytosolic Group IVA and Calcium-Independent Group VIA Phospholipase A2s to Adrenic Acid Mobilization in Murine Macrophages. Biomolecules 2020, 10, 542.
- Brouwers, H.; Jónasdóttir, H.S.; Kuipers, M.E.; Kwekkeboom, J.C.; Auger, J.L.; González-Torres, M.; López-Vicario, C.; Clària, J.; Freysdottir, J.; Hardardottir, I.; et al. Anti-Inflammatory and Proresolving Effects of the Omega-6 Polyunsaturated Fatty Acid Adrenic Acid. J. Immunol. 2020, 205, 2840–2849.
- Harkewicz, R.; Fahy, E.; Andreyev, A.; Dennis, E.A. Arachidonate-Derived Dihomoprostaglandin Production Observed in Endotoxin-Stimulated Macrophage-like Cells. J. Biol. Chem. 2007, 282, 2899–2910.
- Sprecher, H.; Van Rollins, M.; Sun, F.; Wyche, A.; Needleman, P. Dihomo-Prostaglandins and -Thromboxane. A Prostaglandin Family from Adrenic Acid That May Be Preferentially Synthesized in the Kidney. J. Biol. Chem. 1982, 257, 3912–3918.
- Kopf, P.G.; Zhang, D.X.; Gauthier, K.M.; Nithipatikom, K.; Yi, X.Y.; Falck, J.R.; Campbell, W.B. Adrenic Acid Metabolites as Endogenous Endothelium-Derived and Zona Glomerulosa-Derived Hyperpolarizing Factors. Hypertension 2010, 55, 547–554.
- Ross, R. Atherosclerosis—an Inflammatory Disease. N. Engl. J. Med. 1999, 340, 115–126.
- Wong, J.T.; Tran, K.; Pierce, G.N.; Chan, A.C.; O., K.; Choy, P.C. Lysophosphatidylcholine Stimulates the Release of Arachidonic Acid in Human Endothelial Cells. J. Biol. Chem. 1998, 273, 6830–6836.
- Bogatcheva, N.V.; Sergeeva, M.G.; Dudek, S.M.; Verin, A.D. Arachidonic Acid Cascade in Endothelial Pathobiology. Microvasc. Res. 2005, 69, 107–127.
- Badimon, L.; Vilahur, G.; Rocca, B.; Patrono, C. The Key Contribution of Platelet and Vascular Arachidonic Acid Metabolism to the Pathophysiology of Atherothrombosis. Cardiovasc. Res. 2021, 117, 2001–2015.
- Hanasaki, K.; Yamada, K.; Yamamoto, S.; Ishimoto, Y.; Saiga, A.; Ono, T.; Ikeda, M.; Notoya, M.; Kamitani, S.; Arita, H. Potent Modification of Low Density Lipoprotein by Group X Secretory Phospholipase A2 Is Linked to Macrophage Foam Cell Formation. J. Biol. Chem. 2002, 277, 29116–29124.
- Balsinde, J.; Balboa, M.A.; Yedgar, S.; Dennis, E.A. Group V Phospholipase A2-Mediated Oleic Acid Mobilization in Lipopolysaccharide-Stimulated P388D1 Macrophages. J. Biol. Chem. 2000, 275, 4783–4786.
- Sato, H.; Kato, R.; Isogai, Y.; Saka, G.; Ohtsuki, M.; Taketomi, Y.; Yamamoto, K.; Tsutsumi, K.; Yamada, J.; Masuda, S.; et al. Analyses of Group III Secreted Phospholipase A2 Transgenic Mice Reveal Potential Participation of This Enzyme in Plasma Lipoprotein Modification, Macrophage Foam Cell Formation, and Atherosclerosis. J. Biol. Chem. 2008, 283, 23483–33497.
- Karabina, S.A.; Brochériou, I.; Le Naour, G.; Agrapart, M.; Durand, H.; Gelb, M.; Lambeau, G.; Ninio, E. Atherogenic Properties of LDL Particles Modified by Human Group X Secreted Phospholipase A2 on Human Endothelial Cell Function. FASEB J. 2006, 20, 2547–2549.
- Tallima, H.; El Ridi, R. Arachidonic Acid: Physiological Roles and Potential Health Benefits–A Review. J. Adv. Res. 2018, 11, 33–41.
- Guijas, C.; Bermúdez, M.A.; Meana, C.; Astudillo, A.M.; Pereira, L.; Fernández-Caballero, L.; Balboa, M.A.; Balsinde, J. Neutral Lipids Are Not a Source of Arachidonic Acid for Lipid Mediator Signaling in Human Foamy Monocytes. Cells 2019, 8, 941.
- Guijas, C.; Meana, C.; Astudillo, A.M.; Balboa, M.A.; Balsinde, J. Foamy Monocytes Are Enriched in Cis-7-Hexadecenoic Fatty Acid (16:1n-9), a Possible Biomarker for Early Detection of Cardiovascular Disease. Cell Chem. Biol. 2016, 23, 689–699.
- Astudillo, A.M.; Meana, C.; Guijas, C.; Pereira, L.; Lebrero, R.; Balboa, M.A.; Balsinde, J. Occurrence and Biological Activity of Palmitoleic Acid Isomers in Phagocytic Cells. J. Lipid Res. 2018, 59, 237–249.
- Astudillo, A.M.; Meana, C.; Bermúdez, M.A.; Pérez-Encabo, A.; Balboa, M.A.; Balsinde, J. Release of Anti-Inflammatory Palmitoleic Acid and Its Positional Isomers by Mouse Peritoneal Macrophages. Biomedicines 2020, 8, 480.
- Scanferlato, R.; Bortolotti, M.; Sansone, A.; Chatgilialoglu, C.; Polito, L.; De Spirito, M.; Maulucci, G.; Bolognesi, A.; Ferreri, C. Hexadecenoic Fatty Acid Positional Isomers and De Novo PUFA Synthesis in Colon Cancer Cells. Int. J. Mol. Sci. 2019, 20, 832.
- Aryal, P.; Syed, I.; Lee, J.; Patel, R.; Nelson, A.T.; Siegel, D.; Saghatelian, A.; Kahn, B.A. Distinct Biological Activities of Isomers from Several Families of Branched Fatty Acid Esters of Hydroxy Fatty Acids (FAHFAs). J. Lipid Res. 2021, 62, 100108.
- Young, R.S.E.; Bowman, A.P.; Williams, E.D.; Tousignant, K.D.; Bidgood, C.L.; Narreddula, V.R.; Gupta, R.; Marshall, D.L.; Poad, B.L.J.; Nelson, C.C.; et al. Apocryphal FADS2 Activity Promotes Fatty Acid Diversification in Cancer. Cell Rep. 2021, 34, 108738.
- Nomura, D.K.; Morrison, B.E.; Blankman, J.L.; Long, J.Z.; Kinsey, S.G.; Marcondes, M.C.; Ward, A.M.; Hahn, Y.K.; Lichtman, A.H.; Conti, B.; et al. Endocannabinoid Hydrolysis Generates Brain Prostaglandins That Promote Neuroinflammation. Science 2011, 334, 809–813.
- Grabner, G.F.; Eichmann, T.O.; Wagner, B.; Gao, Y.; Farzi, A.; Taschler, U.; Radner, F.P.; Schweiger, M.; Lass, A.; Holzer, P.; et al. Deletion of Monoglyceride Lipase in Astrocytes Attenuates Lipopolysaccharide-Induced Neuroinflammation. J. Biol. Chem. 2016, 291, 913–923.
- Schlager, S.; Vujic, N.; Korbelius, M.; Duta-Mare, M.; Dorow, J.; Leopold, C.; Rainer, S.; Wegscheider, M.; Reicher, H.; Ceglarek, U.; et al. Lysosomal Lipid Hydrolysis Provides Substrates for Lipid Mediator Synthesis in Murine Macrophages. Oncotarget 2017, 8, 40037–40051.
- Dichlberger, A.; Schlager, S.; Maaninka, K.; Schneider, W.J.; Kovanen, P.T. Adipose Triglyceride Lipase Regulates Eicosanoid Production in Activated Human Mast Cells. J. Lipid Res. 2014, 55, 2471–2478.
- Schlager, S.; Goeritzer, M.; Jandl, K.; Frei, R.; Vujic, N.; Kolb, D.; Strohmaier, H.; Dorow, J.; Eichmann, T.O.; Rosenberger, A.; et al. Adipose Triglyceride Lipase Acts on Neutrophil Lipid Droplets to Regulate Substrate Availability for Lipid Mediator Synthesis. J. Leukoc. Biol. 2015, 98, 837–850.
- Dennis, E.A.; Cao, J.; Hsu, Y.H.; Magrioti, V.; Kokotos, G. Phospholipase A2 Enzymes: Physical Structure, Biological Function, Disease Implication, Chemical Inhibition, and Therapeutic Intervention. Chem. Rev. 2011, 111, 6130–6185.
- Murakami, M. Novel Functions of Phospholipase A2s: Overview. Biochim. Biophys. Acta 2019, 1864, 763–765.
- Mouchlis, V.D.; Dennis, E.A. Phospholipase A2 Catalysis and Lipid Mediator Lipidomics. Biochim. Biophys. Acta 2019, 1864, 766–771.
- Kita, Y.; Shindou, H.; Shimizu, T. Cytosolic Phospholipase A2 and Lysophospholipid Acyltransferases. Biochim. Biophys. Acta 2019, 1864, 838–845.
- Turk, J.; White, T.D.; Nelson, A.J.; Lei, X.; Ramanadham, S. iPLA2β and Its Role in Male Fertility, Neurological Disorders, Metabolic Disorders, and Inflammation. Biochim. Biophys. Acta 2019, 1864, 846–860.
- Murakami, M.; Sato, H.; Miki, Y.; Yamamoto, K.; Taketomi, Y. A New Era of Secreted Phospholipase A2. J. Lipid Res. 2015, 56, 1248–1261.
- Murakami, M.; Sato, H.; Taketomi, Y. Updating Phospholipase A2 Biology. Biomolecules 2020, 10, 1457.
- White, T.D.; Almutairi, A.; Tusing, Y.G.; Lei, X.; Ramanadham, S. The Impact of the Ca2+-Independent Phospholipase A2β on Immune Cells. Biomolecules 2021, 11, 577.
- Dabral, D.; van den Bogaart, G. The Roles of Phospholipase A2 in Phagocytes. Front. Cell Dev. Biol. 2021, 9, 673502.
- Peng, Z.; Chang, Y.; Fan, J.; Ji, W.; Su, C. Phospholipase A2 Superfamily in Cancer. Cancer Lett. 2021, 497, 165–177.
- Sun, G.Y.; Geng, X.; Teng, T.; Yang, B.; Appenteng, M.K.; Greenlief, C.M. Dynamic Role of Phospholipases A2 in Health and Diseases in the Central Nervous System. Cells 2021, 10, 2963.
- Pindado, J.; Balsinde, J.; Balboa, M.A. TLR3-Dependent Induction of Nitric Oxide Synthase in RAW 264.7 Macrophage-like Cells Via a Cytosolic Phospholipase A2/Cyclooxygenase-2 Pathway. J. Immunol. 2007, 179, 4821–4828.
- Astudillo, A.M.; Rodríguez, J.P.; Guijas, C.; Rubio, J.M.; Balboa, M.A.; Balsinde, J. Choline Glycerophospholipid-Derived Prostaglandins Attenuate TNFα Gene Expression in Macrophages Via a cPLA2α/COX-1 Pathway. Cells 2021, 10, 447.
- Mouchlis, V.D.; Chen, Y.; McCammon, J.A.; Dennis, E.A. Membrane Allostery and Unique Hydrophobic Sites Promote Enzyme Substrate Specificity. J. Am. Chem. Soc. 2018, 140, 3285–3291.
- Gil-de-Gómez, L.; Astudillo, A.M.; Guijas, C.; Magrioti, V.; Kokotos, G.; Balboa, M.A.; Balsinde, J. Cytosolic Group IVA and Calcium-Independent Group VIA Phospholipase A2s Act on Distinct Phospholipid Pools in Zymosan-Stimulated Mouse Peritoneal Macrophages. J. Immunol. 2014, 192, 752–762.
- Lebrero, P.; Astudillo, A.M.; Rubio, J.M.; Fernández-Caballero, J.; Kokotos, G.; Balboa, M.A.; Balsinde, J. Cellular Plasmalogen Content Does Not Influence Arachidonic Acid Levels or Distribution in Macrophages: A Role for Cytosolic Phospholipase A2γ in Phospholipid Remodeling. Cells 2019, 8, 799.
- Gil-de-Gómez, L.; Monge, P.; Rodríguez, J.P.; Astudillo, A.M.; Balboa, M.A.; Balsinde, J. Phospholipid Arachidonic acid Remodeling during Phagocytosis in Mouse Peritoneal Macrophages. Biomedicines 2020, 8, 274.
- Murakami, M.; Lambeau, G. Emerging Roles of Secreted Phospholipase A2 Enzymes: An Update. Biochimie 2013, 95, 43–50.
- Samuchiwal, S.K.; Balestrieri, B. Harmful and Protective Roles of Group V Phospholipase A2: Current Perspectives and Future Directions. Biochim. Biophys. Acta 2019, 1864, 819–826.
- Balboa, M.A.; Shirai, Y.; Gaietta, G.; Ellisman, M.H.; Balsinde, J.; Dennis, E.A. Localization of Group V Phospholipase A2 in Caveolin-Enriched Granules in Activated P388D1 Macrophage-like Cells. J. Biol. Chem. 2003, 278, 48059–48065.
- Bingham, C.O.; Fijneman, R.J.; Friend, D.S.; Goddeau, R.P.; Rogers, R.A.; Austen, K.F.; Arm, J.P. Low Molecular Weight Group IIA and Group V Phospholipase A2 Enzymes Have Different Intracellular Locations in Mouse Bone Marrow-Derived Mast Cells. J. Biol. Chem. 1999, 274, 31476–31484.
- Ruipérez, V.; Astudillo, M.A.; Balboa, M.A.; Balsinde, J. Coordinate Regulation of TLR-Mediated Arachidonic Acid Mobilization in Macrophages by Group IVA and Group V Phospholipase A2s. J. Immunol. 2009, 182, 3877–3883.
- Balboa, M.A.; Pérez, R.; Balsinde, J. Amplification Mechanisms of Inflammation: Paracrine Stimulation of Arachidonic Acid Mobilization by Secreted Phospholipase A2 Is Regulated by Cytosolic Phospholipase A2-Derived Hydroperoxyeicosatetraenoic Acid. J. Immunol. 2003, 171, 989–994.
- Kikawada, E.; Bonventre, J.V.; Arm, J.P. Group V Secretory PLA2 Regulates TLR2-Dependent Eicosanoid Generation in Mouse Mast Cells through Amplification of ERK and cPLA2α Activation. Blood 2007, 110, 561–567.
- Murakami, M.; Miki, Y.; Sato, H.; Murase, R.; Taketomi, Y.; Yamamoto, K. Group IID, IIE, IIF and III Secreted Phospholipase A2s. Biochim. Biophys. Acta 2019, 1864, 803–818.
- Miki, Y.; Yamamoto, K.; Taketomi, Y.; Sato, H.; Shimo, K.; Kobayashi, T.; Ishikawa, Y.; Ishii, T.; Nakanishi, H.; Ikeda, K.; et al. Lymphoid Tissue Phospholipase A2 Group IID Resolves Contact Hypersensitivity by Driving Antiinflammatory Lipid Mediators. J. Exp. Med. 2013, 210, 1217–1234.
- Ait-Oufella, H.; Herbin, O.; Lahoute, C.; Coatrieux, C.; Loyer, X.; Joffre, J.; Laurans, L.; Ramkhelawon, B.; Blanc-Brude, O.; Karabina, S.; et al. Group X Secreted Phospholipase A2 Limits the Development of Atherosclerosis in LDL Receptor-Null Mice. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 466–473.
- Murase, R.; Sato, H.; Yamamoto, K.; Ushida, A.; Nishito, Y.; Ikeda, K.; Kobayashi, T.; Yamamoto, T.; Taketomi, Y.; Murakami, M. Group X Secreted Phospholipase A2 Releases ω3 Polyunsaturated Fatty Acids, Suppresses Colitis, and Promotes Sperm Fertility. J. Biol. Chem. 2016, 291, 6895–6911.
- Murphy, R.C.; Folco, G. Lysophospholipid Acyltransferases and Leukotriene Biosynthesis: Intersection of the Lands Cycle and the Arachidonate PI Cycle. J. Lipid Res. 2019, 60, 219–226.
- Patton-Vogt, J.; de Kroon, A.I.P.M. Phospholipid Turnover and Acyl Chain Remodeling in the Yeast ER. Biochim. Biophys. Acta 2020, 1865, 158462.
- Yamashita, A.; Hayashi, Y.; Nemoto-Sasaki, Y.; Ito, M.; Oka, S.; Tanikawa, T.; Waku, K.; Sugiura, T. Acyltransferases and Transacylases That Determine the Fatty Acid Composition of Glycerolipids and the Metabolism of Bioactive Lipid Mediators in Mammalian Cells and Model Organisms. Prog. Lipid Res. 2014, 53, 18–81.
- Yamashita, A.; Hayashi, Y.; Matsumoto, N.; Nemoto-Sasaki, Y.; Koizumi, T.; Inagaki, Y.; Oka, S.; Tanikawa, T.; Sugiura, T. Coenzyme-A-Independent Transacylation System; Possible Involvement of Phospholipase A2 in Transacylation. Biology 2017, 6, 23.
- Ghosh, M.; Tucker, D.E.; Burchett, S.A.; Leslie, C.C. Properties of the Group IV Phospholipase A2 Family. Prog. Lipid Res. 2006, 45, 487–510.
- Gil-de-Gómez, L.; Astudillo, A.M.; Lebrero, P.; Balboa, M.A.; Balsinde, J. Essential Role for Ethanolamine Plasmalogen Hydrolysis in Bacterial Lipopolysaccharide Priming of Macrophages for Enhanced Arachidonic Acid Release. Front. Immunol. 2017, 8, 1251.
- Rubio, J.M.; Astudillo, A.M.; Casas, J.; Balboa, M.A.; Balsinde, J. Regulation of Phagocytosis in Macrophages by Membrane Ethanolamine Plasmalogens. Front. Immunol. 2018, 9, 1723.
