Malaria burden has severe impact on the world. Several arsenals, including the use of antimalarials, are in place to curb the malaria burden. Limited access to drugs ensures that patients do not get the right doses of the antimalarials in order to have an effective plasma concentration to kill the malaria parasites, which leads to treatment failure and overall reduction in malaria control via increased transmission rate.
- malaria
- drug-pressure
- limited-access
- antimalarials
- treatment
1. Introduction
2. Overview of the Current Antimalarial Portfolio
|
Antimalarials |
Introduction Date |
Resistance Date |
Genetic Marker |
Mechanism of Action |
Mechanism of Resistance |
References |
|
|---|---|---|---|---|---|---|---|
Region Used |
Region of Reported ACT Failure |
||||||
|
Aryl-amino alcohol |
|||||||
|
Artemether-lumefantrine (AL) |
Africa, Americas and Middle East |
Burkina Faso, Cambodia, Lao People’s Democratic Republic, Thailand and Vietnam |
|||||
|
Quinine |
|||||||
|
Dihydroartemisinin-piperaquine (DHA-PPQ) | 1820 |
1910 |
Southeast Asia, China and Africa Pfmdr1 |
Inhibition of heme detoxification |
Gene amplification and mutation leading to drug efflux and/or non-binding to target site |
Cambodia, Lao People’s Democratic Republic, Thailand and Vietnam |
|
|
Mefloquine |
1977 |
1982 |
|||||
|
Artesunate-amodiaquine (AS-AQ) |
West Africa |
Indonesia, Cambodia |
Pfmdr1 |
||||
|
Lumefantrine |
|||||||
|
Artesunate-mefloquine (AS-MQ) | 1976 |
Southeast Asia and Americas |
Cambodia, Lao People’s Democratic Republic, Thailand and Vietnam |
Pfmdr1 |
|||
|
4-aminoquinolines |
|||||||
|
Artesunate-sulfadoxine-pyrimethamine (AS-SP) |
Southeast Asia, Middle-East and South America |
Northeastern India, Somalia and Sudan |
Chloroquine |
1945 |
1957 |
Pfcrt, Pfmdr |
Inhibition of heme detoxification and redox cycling of heme to and fro the cytosol |
|
Artesunate—pyronaridine (AS-PY) |
Southeast Asia |
Cambodia, Vietnam | Gene mutation: efflux of molecules from food vacuole | ||||
|
Amodiaquine |
1948 |
1990s |
Pfcrt, Pfmdr |
||||
|
Bis-quinoline |
|||||||
|
Piperaquine |
1960s |
2010 |
Pfcrt, Pfplasmepsin 2-3 copy number |
Inhibition of heme detoxifica-tion and redox cycling of heme to and fro the cytosol |
Gene amplification |
||
|
Naphthyridine |
|||||||
|
Pyronaridine |
1980 |
- |
Inhibition of heme detoxification |
||||
|
Antifolates |
|||||||
|
Sulfadoxine |
1937 |
1960s |
Pfdhps |
Inhibition of folate metabolism and DNA replication |
Gene mutation leading to drug binding site modification |
||
|
Proguanil/cycloguanil |
1948 |
1949 |
Pfdhfr |
||||
|
Pyrimethamine |
1952 |
1960s |
|||||
|
Endoperoxide |
|||||||
|
Artemisinin and its derivatives (DHA, ATS, ATM) |
1972 |
2008 |
PfKelch13 | ||||
3. Limited Access to Drugs
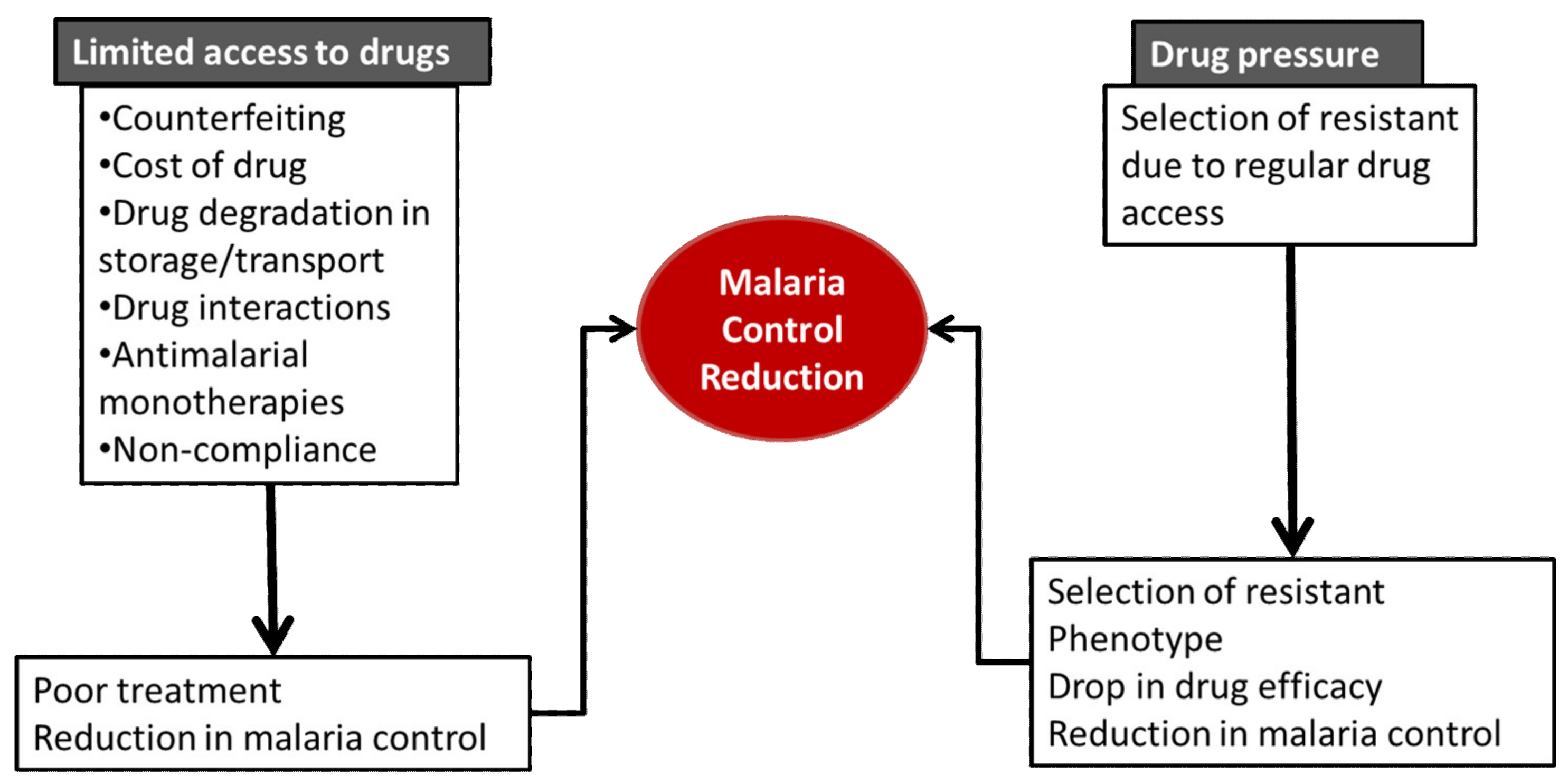
3.1. Counterfeiting/Drug Quality
3.2. Cost of Drug
3.3. Drug Storage/Transportation
3.4. Drug Interactions
|
Food/Drug |
Class |
Probable Mechanism of Interaction |
Consequences |
|||
|---|---|---|---|---|---|---|
|
Indinavir, nelfinavir |
Antiretroviral |
Inhibits CYP3A4 |
May increase concentrations of ART and LUM |
|||
|
Imatinib |
Anticancer |
Inhibits Syk, Lyn, Bcr-Abl |
Decrease artemisinin concentration and accelerate ART efficacy |
|||
|
Ritonavir |
Antiretroviral |
Inhibits CYP2D6 and CYP3A4 |
May increase concentrations of ART and LUM |
|||
|
Ketoconazole |
Antifungal |
Inhibits CYP3A4 |
Shown to cause modest increase in concentration of ART and LUM |
|||
|
Fluconazole |
Antifungal |
Inhibits CYP3A4 |
May cause increase in concentration of ART and LUM |
|||
Inhibition of heme detoxification and C-C radical formation | ||||||
Entry into quiescent state | ||||||
[ | ] | [ | ] | |||
|
Hydroxynaphtoquinones |
||||||
|
Atovaquone |
1996 |
1996 |
Pfcytb |
Competitive inhibition of Complex iii of the ETC |
Modification of binding site on Complex iii/cytochrome b |
|
|
Tetracycline antibiotic |
||||||
|
Doxycycline |
1967 |
SNPs in Pfmdt and PftetQ |
Inhibition of protein, nucleotide and deoxynucleotide synthesis |
Not yet described |
||
|
ACTs | |||
|---|---|---|---|
Rifampicin, isoniazid | |||
Anti-tuberculosis |
Induces CYP3A4 |
May decrease concentrations of ART and LUM |
|
|
Nevirapine, efavirenz |
Antiretrovirals |
Induces CYP3A4 |
May decrease concentrations of ART and LUM |
|
Phentytoin/phenobarbital /carbamazepine |
Anticonvulsants |
Induces CYP3A4 |
May decrease concentrations of ART and LUM |
Adapted from [46,47,48][43][44][45]. ART = artemisinin, LUM = lumefantrine, CYP = cytochrome P450.
3.5. Use of Antimalarials as Monotherapies
3.6. Hoarding of Drugs by Corrupt Officials
References
- World Health Organization. World Malaria Report 2019; WHO: Geneva, Switzerland, 2019; ISBN 9789241565721.
- WHO. World Malaria Report 2020: 20 Years of Global Progress and Challenges; WHO: Geneva, Switzerland, 2020.
- Payne, D. Spread of chloroquine resistance in Plasmodium falciparum. Parasitol. Today 1987, 3, 241–246.
- Noedl, H.; Se, Y.; Schaecher, K.; Smith, B.L.; Socheat, D.; Fukuda, M.M. Evidence of artemisinin-resistant malaria in Western Cambodia. N. Engl. J. Med. 2008, 359, 2619–2620.
- Bloland, P.B. Drug Resistance in Malaria (WHO/CDS/CSR/DRS/2001.4). Available online: https://www.who.int/csr/resources/publications/drugresist/malaria.pdf (accessed on 20 January 2021).
- Willcox, M.L.; Bodeker, G. Traditional herbal medicines for malaria. Br. Med. J. 2004, 329, 1156–1159.
- Nzila, A. The past, present and future of antifolates in the treatment of Plasmodium falciparum infection. J. Antimicrob. Chemother. 2006, 57, 1043–1054.
- Robert, A.; Benoit-Vical, F.; Claparols, C.; Meunier, B. The antimalarial drug artemisinin alkylates heme in infected mice. Proc. Natl. Acad. Sci. USA 2005, 102, 13676–13680.
- Sullivan, D.J.; Matile, H.; Ridley, R.G.; Goldberg, D.E. A common mechanism for blockade of heme polymerization by antimalarial quinolines. J. Biol. Chem. 1998, 273, 31103–31107.
- Golden, E.B.; Cho, H.Y.; Hofman, F.M.; Louie, S.G.; Schönthal, A.H.; Chen, T.C. Quinoline-based antimalarial drugs: A novel class of autophagy inhibitors. Neurosurg. Focus 2015, 38, E12.
- Sidhu, A.B.S.; Valderramos, S.G.; Fidock, D.A. pfmdr1 mutations contribute to quinine resistance and enhance mefloquine and artemisinin sensitivity in Plasmodium falciparum. Mol. Microbiol. 2005, 57, 913–926.
- Price, R.N.; Uhlemann, A.C.; Brockman, A.; McGready, R.; Ashley, E.; Phaipun, L.; Patel, R.; Laing, K.; Looareesuwan, S.; White, N.J.; et al. Mefloquine resistance in Plasmodium falciparum and increased pfmdr1 gene copy number. Lancet 2004, 364, 438–447.
- Holmgren, G.; Hamrin, J.; Svärd, J.; Mårtensson, A.; Gil, J.P.; Björkman, A. Selection of pfmdr1 mutations after amodiaquine monotherapy and amodiaquine plus artemisinin combination therapy in East Africa. Infect. Genet. Evol. 2007, 7, 562–569.
- Pickard, A.L.; Wongsrichanalai, C.; Purfield, A.; Kamwendo, D.; Emery, K.; Zalewski, C.; Kawamoto, F.; Miller, R.S.; Meshnick, S.R. Resistance to antimalarials in Southeast Asia and genetic polymorphisms in pfmdr1. Antimicrob. Agents Chemother. 2003, 47, 2418–2423.
- Martin, R.E.; Marchetti, R.V.; Cowan, A.I.; Howitt, S.M.; Bröer, S.; Kirk, K. Chloroquine transport via the malaria parasite’s chloroquine resistance transporter. Science 2009, 325, 1680–1682.
- Beshir, K.; Sutherland, C.J.; Merinopoulos, I.; Durrani, N.; Leslie, T.; Rowland, M.; Hallett, R.L. Amodiaquine resistance in Plasmodium falciparum malaria in Afghanistan is associated with the pfcrt SVMNT allele at codons 72 to 76. Antimicrob. Agents Chemother. 2010, 54, 3714–3716.
- Witkowski, B.; Duru, V.; Khim, N.; Ross, L.S.; Saintpierre, B.; Beghain, J.; Chy, S.; Kim, S.; Ke, S.; Kloeung, N.; et al. A surrogate marker of piperaquine-resistant Plasmodium falciparum malaria: A phenotype–genotype association study. Lancet Infect. Dis. 2017, 17, 174–183.
- Dhingra, S.K.; Small-Saunders, J.L.; Ménard, D.; Fidock, D.A. Plasmodium falciparum resistance to piperaquine driven by PfCRT. Lancet Infect. Dis. 2019, 19, 1168–1169.
- Croft, S.L.; Duparc, S.; Arbe-Barnes, S.J.; Craft, J.C.; Shin, C.S.; Fleckenstein, L.; Borghini-Fuhrer, I.; Rim, H.J. Review of pyronaridine anti-malarial properties and product characteristics. Malar. J. 2012, 11, 270.
- Hyde, J.E. Drug-resistant malaria—An insight. FEBS J. 2007, 274, 4688–4698.
- Staines, H.M.; Burrow, R.; Teo, B.H.Y.; Ster, I.C.; Kremsner, P.G.; Krishna, S. Clinical implications of Plasmodium resistance to atovaquone/proguanil: A systematic review and meta-analysis. J. Antimicrob. Chemother. 2018, 73, 581–595.
- Ariey, F.; Witkowski, B.; Amaratunga, C.; Beghain, J.; Langlois, A.C.; Khim, N.; Kim, S.; Duru, V.; Bouchier, C.; Ma, L.; et al. A molecular marker of artemisinin-resistant Plasmodium falciparum malaria. Nature 2014, 505, 50–55.
- Musset, L.; Bouchaud, O.; Matheron, S.; Massias, L.; Le Bras, J. Clinical atovaquone-proguanil resistance of Plasmodium falciparum associated with cytochrome b codon 268 mutations. Microbes Infect. 2006, 8, 2599–2604.
- Conrad, M.D.; Rosenthal, P.J. Antimalarial drug resistance in Africa: The calm before the storm? Lancet Infect. Dis. 2019, 19, e338–e351.
- Gaillard, T.; Madamet, M.; Pradines, B. Tetracyclines in malaria. Malar. J. 2015, 14, 445.
- Dipanjan, B.; Shivaprakash, G.; Balaji, O. Triple Combination Therapy and Drug Cycling—Tangential Strategies for Countering Artemisinin Resistance. Curr. Infect. Dis. Rep. 2017, 19, 25.
- Gansané, A.; Moriarty, L.F.; Ménard, D.; Yerbanga, I.; Ouedraogo, E.; Sondo, P.; Kinda, R.; Tarama, C.; Soulama, E.; Tapsoba, M.; et al. Anti-malarial efficacy and resistance monitoring of artemether-lumefantrine and dihydroartemisinin-piperaquine shows inadequate efficacy in children in Burkina Faso, 2017–2018. Malar. J. 2021, 20, 48.
- WHO Artemisinin Resistance and Artemisinin-Based Combination Therapy Efficacy (Status Report—August 2018). Available online: https://apps.who.int/iris/bitstream/handle/10665/274362/WHO-CDS-GMP-2018.18-eng.pdf?ua=1 (accessed on 26 December 2020).
- WHO Report on Antimalarial Drug Efficacy, Resistance and Response: 10 Years of Surveillance (2010–2019). Available online: https://www.who.int/publications/i/item/9789240012813 (accessed on 27 December 2020).
- McKeage, K.; Scott, L.J.; Borrmann, S.; De Vries, P.J.; Hutchinson, D.B.A.; Looareesuwan, S.; Nosten, F.; Price, R.; Shanks, G.D. Atovaquone/proguanil: A review of its use for the prophylaxis of Plasmodium falciparum malaria. Drugs 2003, 63, 597–623.
- Deloron, P.; Bertin, G.; Briand, V.; Massougbodji, A.; Cot, M. Sulfadoxine/Pyrimethamine Intermittent Preventive Treatment for Malaria during Pregnancy. Emerg. Infect. Dis. 2010, 16, 1666.
- FDA. Guidance for Industry: Q8(R2) Pharmaceutical Development|FDA. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/q8r2-pharmaceutical-development (accessed on 8 June 2021).
- WHO. Quality Assurance of Pharmaceuticals: A Compendium of Guidelines and Related Materials. Available online: https://www.who.int/medicines/areas/quality_safety/quality_assurance/QualityAssurancePharmVol2.pdf (accessed on 24 June 2021).
- WWARN. Access to Quality Antimalarials—Determining the Scale of the Problem. Worldwide Antimalarial Resistance Network. Available online: https://www.wwarn.org/news/news-articles/access-quality-antimalarials-determining-scale-problem (accessed on 8 June 2021).
- WHO. 1 in 10 Medical Products in Developing Countries Is Substandard or Falsified. Available online: https://www.who.int/news-room/detail/28-11-2017-1-in-10-medical-products-in-developing-countries-is-substandard-or-falsified (accessed on 3 July 2021).
- Amin, A.A.; Kokwaro, G.O. Antimalarial drug quality in Africa. J. Clin. Pharm. Ther. 2007, 32, 145–150.
- Renschler, J.P.; Walters, K.M.; Newton, P.N.; Laxminarayan, R. Estimated under-five deaths associated with poor-quality antimalarials in sub-Saharan Africa. Am. J. Trop. Med. Hyg. 2015, 92, 119–126.
- Spaan, E.; Mathijssen, J.; Tromp, N.; McBain, F.; ten Have, A.; Baltussen, R. The impact of health insurance in Africa and Asia: A systematic review. Bull. World Health Organ. 2012, 90, 685–692.
- WHO. World Health Statistics 2010. Available online: https://apps.who.int/iris/handle/10665/44292 (accessed on 9 June 2021).
- McIntyre, D.; Thiede, M.; Dahlgren, G.; Whitehead, M. What are the economic consequences for households of illness and of paying for health care in low- and middle-income country contexts? Soc. Sci. Med. 2006, 62, 858–865.
- Briscoe, C.J.; Hage, D.S. Factors affecting the stability of drugs and drug metabolites in biological matrices. Bioanalysis 2009, 1, 205–220.
- Tarning, J.; Hoglund, R.M. Clinically Relevant Drug Interactions for Malaria. In Encyclopedia of Malaria; Kremsner, P.G., Krishna, S., Eds.; Springer: New York, NY, USA, 2019.
- Kokwaro, G.; Mwai, L.; Nzila, A. Artemether/lumefantrine in the treatment of uncomplicated falciparum malaria. Expert Opin. Pharmacother. 2007, 8, 75–94.
- Adedeji, W.A.; Balogun, T.; Fehintola, F.A.; Morse, G.D. Drug-Drug Interactions of Antimalarial Drugs. In Drug Interactions in Infectious Diseases: Antimicrobial Drug Interactions; Pai, M.P., Kiser, J.J., Gubbins, P.O., Rodvold, K.A., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 503–514.
- Tsamesidis, I.; Reybier, K.; Marchetti, G.; Pau, M.C.; Virdis, P.; Fozza, C.; Nepveu, F.; Low, P.S.; Turrini, F.M.; Pantaleo, A. Syk Kinase Inhibitors Synergize with Artemisinins by Enhancing Oxidative Stress in Plasmodium falciparum-Parasitized Erythrocytes. Antioxidants 2020, 9, 753.
- White, N. Antimalarial drug resistance and combination chemotherapy. Philos. Trans. R. Soc. B Biol. Sci. 1999, 354, 739–749.
