Large areas in the northern hemisphere are covered by extensive wetlands, which represent a complex mosaic of raised bogs, eutrophic fens, and aapa mires all in proximity to each other. Aapa mires differ from other types of wetlands by their concave surface, heavily watered by the central part, as well as by the presence of large-patterned string-flark complexes. The microbial communities in raised strings were clearly distinct from those in submerged flarks. Strings were dominated by the Alpha- and Gammaproteobacteria. Other abundant groups were the Acidobacteriota, Bacteroidota, Verrucomicrobiota, Actinobacteriota, and Planctomycetota. Archaea accounted for only 0.4% of 16S rRNA gene sequences retrieved from strings. By contrast, they comprised about 22% of all sequences in submerged flarks and mostly belonged to methanogenic lineages. Methanotrophs were nearly absent. Other flark-specific microorganisms included the phyla Chloroflexi, Spirochaetota, Desulfobacterota, Beijerinckiaceae- and Rhodomicrobiaceae-affiliated Alphaproteobacteria, and uncultivated groups env.OPS_17 and vadinHA17 of the Bacteroidota. Such pattern probably reflects local anaerobic conditions in the submerged peat layers in flarks.
1. Introduction
Wetlands are among the most productive ecosystems on Earth, performing various ecosystem functions important for human life and sustainable development
[1]. Wetlands play an important role in the global water balance, ensuring the accumulation, long-term conservation and releasing of water, thereby maintaining the resilience of water flows to short-term fluctuations in the level of precipitation
[1][2][1,2]. Wetlands are also important for their high nutrient recycling capacities and significant contributions to both carbon accumulation and storage and greenhouse gas emissions
[3][4][3,4]. The total area of wetlands worldwide is about 5.5 million km
2 [4][5][4,5], of which the peat accumulating wetlands (peatlands) account for about 4 million km
2. About 80% of peatlands are located in zones with a temperate and cold climate in the northern hemisphere, mainly in Russia, Canada, the United States and Scandinavia
[5][6][5,6].
Peatlands differ in several types depending on the water source and the type of vegetation. The two most contrasting types of peatlands are raised bogs, fed by rainwater, and eutrophic fens, which are primarily filled with groundwater and runoff
[3][7][3,7]. Raised bogs are peat bogs dominated by
Sphagnum mosses. Generally, raised bogs are highly acidic (pH around 4) and nutrient-poor. Microbial communities of raised bogs were studied using both cultivation-based and molecular methods, including fluorescence in situ hybridization, high-throughput sequencing of 16S rRNA gene fragments, metagenomics and metatrancriptomics
[8][9][10][11][12][13][14][15][16][17][8,9,10,11,12,13,14,15,16,17]. The microbial communities of this type of peatlands are usually dominated by bacteria of the phyla
Acidobacteriota and
Proteobacteria (classes
Alpha- and
Gammaproteobacteria);
Verrucomicrobiota and
Planctomycetota also make up a significant share of the communities
[8][9][11][12][13][14][15][16][17][8,9,11,12,13,14,15,16,17].
Eutrophic fens are fed primarily by groundwater. They are generally less acidic and more nutrient-rich than bogs, with sedges and grasses being the main vegetation type. Microbial communities of fens are characterized to a lesser extent; most studies were focused on microorganisms participating in the methane cycle
[8][18][19][8,18,19]. Microbial communities of eutrophic fens are more diverse and differ greatly in composition from raised bogs
[11]. In these communities, the dominant groups are
Chloroflexi of the class
Anaerolineae, some lineages of
Acidobacteriota and
Betaproteobacteria, and
Planctomycetota of the uncultivated OM190 group
[20].
Aapa mires is a term used for large patterned or ribbed fens
[21]. Mires of this type differ from other types of wetlands by their concave surface, heavily watered by the central part, as well as by the presence of string-flark complexes
[22][23][24][25][22,23,24,25]. The latter means that strings and flarks have significant differences in origin, trophic status, and the type of vegetation. So, the strings (elevated forms of microrelief) pass in their development sequentially eutrophic, mesotrophic, and oligotrophic stages and currently have dwarf shrub-
Sphagnum and, as a rule, forested communities, underlain by raised peats. At the same time, flarks throughout their development are in the eutrophic stage; therefore, they have herbal, herbal-
Hypnum or herbal-
Hypnum-Sphagnum communities on fen peats. The regular recurrence of such strings and flarks (provided that the strings are located perpendicular to the water flow direction) creates aapa complexes that provide the characteristic landscape to this type of wetlands
[25][26][27][28][29][25,26,27,28,29].
Aapa mires have a pan-boreal distribution range
[30]. In northern Europe, the prevalent territory for their distribution is the northern part of Fennoscandia, where aapa is the dominant zonal type of mire massifs and occupies tens of thousands of square kilometres
[26]. In the European part of Russia, aapa mire massifs belong to one of three groups based on their morphology and composition of their vegetation: (1) northern European forest-tundra (Lapland) aapa; (2) Karelian ring (boreal Fennoscandian) aapa; and (3) Onega-Pechora (north-eastern) aapa. The southern boundary of the sporadic distribution of aapa mires in the north of European Russia lies between 60° N and 61° N, near the southern boundary of the middle taiga subzone
[31].
Russia accounts for a significant share of all the wetlands in the world. Large areas of wetlands are located in the region of Western Siberia, but in the European part of the country their area is about 15 million hectares
[29]. Many large mire regions in North European Russia include closely located peatlands of various types, mainly raised bogs and eutrophic fens, and less often aapa type wetlands. While microbial communities of raised bogs and eutrophic fens located in this region were characterized in several studies
[13][15][20][13,15,20], there is no such information on the aapa-mires.
2. Study Site
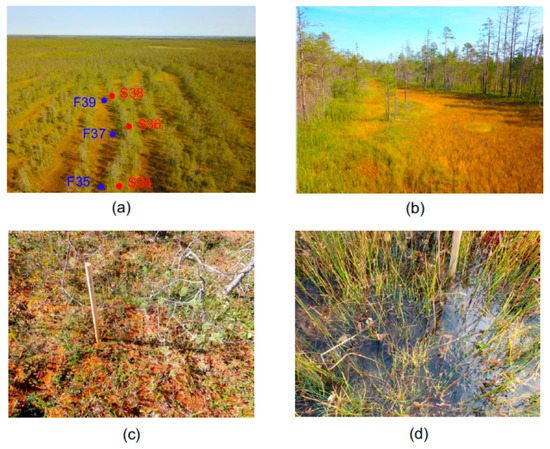
The object of this study was Piyavochnoe mire located in the north-west of Vologda region of North European Russia in the southern part of the middle taiga subzone. This is a large (80 km
2) mire system composed of several raised bogs, aapa-mires and fen massifs, and a number of intramire primary lakes
[31]. In the Piyavochnoe mire, the mire massif of the aapa type (coordinates 60.475 N, 36.504 E) is located in a separate depression and has a pronounced string-flark microrelief (alternation of forested
Sphagnum ridges, grassed depression and low
Sphagnum carpets located between them) in the central part (
Figure 1). This aapa mire belongs to the Onega–Pechora aapa group, but has some features characteristic of the Karelian ring aapa mires
[31].
Figure 1. Aapa-type mire in Piyavochnoe mire system. (a) Aerial view, approximate positions of sampling sites are marked; (b) surface view showing a flark in center, and forest-covered strings on left and right sides; (c) sampling site in string; (d) sampling site in flark.
The strings are relatively young in origin
[31], have a height of 0.20–0.35 m, a width of 5–20 m, and are stretched over the entire width of the massif (400–600 m). They are occupied by pine-grass-shrub-
Sphagnum oligotrophic plant communities.
The flarks are completely closed, watered (depth 0.1–0.3 m, width varies from 1–3 to 25–30 m, and even 30–55 m with a length of 150–400 m), and occupied by hydrophilic herb-
Hypnum vegetation. Among the herbs,
Carex lasiocarpa and
Menyanthes trifoliata dominate;
Rhynchospora alba, Trichophorum alpinum,
Scheuchzeria palustris are also present;
Utricularia intermedia and
U. minor are abundant under water. Unlike strings, mosses do not form a continuous cover here; the dominant moss is
Scorpidium scorpioides submerged in water. The plant species were identified using the guide to vascular plants of the North-West of Russia
[32].
3. Main Characteristics of Peat in Strings and Flarks
The analyzed peat samples obtained from string and flark sites showed a number of important differences regarding their chemical composition (
Table 1). First, at the string sites, the groundwater level was at a depth of 10 to 20 cm, and at the flark sites the peat samples were completely submerged in water. The water in string samples had lower pH (4.6–5.2) than in flarks (5.5–5.9). While the total organic carbon contents in peat collected from string and flark sites were similar (97–98%), the flark water contained more ammonium as the strings, although this difference was not statistically significant (
p = 0.10).
Table 1. Physical and chemical characteristics of sampling sites.
| Sample ID |
S-34 |
S-36 |
S-38 |
F-35 |
F-37 |
F-39 |
| Sample type |
string |
string |
string |
flark |
flark |
flark |
| Water level (cm) * |
−17…−19 |
−10…−12 |
−11…−13 |
+9…+10 |
+8…+10 |
+7…+9 |
| 1210 |
| 1340 |
| 1190 |
| 1070 |
| 1130 |
| 1110 |
Plant community
(dominant species) |
Pinus sylvestris–Empetrum hermaphroditum–Sphagnum fuscum |
Carex lasiocarpa–Scorpidium scorpioides |
| Vegetation coverage |
97–99% |
50–60% |
* minus sign means the depth of groundwater; plus sign—depth of water covering surface; ranges of values are shown; ** chemical parameters for which differences between strings and flarks were statistically significant according to ANOVA test (p < 0.05).
The concentrations of nitrate were an order of magnitude lower than of ammonium. The content of sulfate in string peat by far exceeded that in peat from flarks. The concentrations of phosphorous and magnesium in peat from strings were higher than those in flarks, while the content of iron and calcium did not differ significantly (
Table 1).
43. Diversity of Microbial Communities
To characterize the compositions of microbial communities, between 12,421 and 59,099 sequences of 16S rRNA gene fragments (655,536 in total) were determined for 18 analyzed peat samples. As a result of clustering the obtained sequences, 5264 bacterial and 234 archaeal OTUs were identified at the level of 97% sequence identity. The rarefaction curve of the observed OTUs approached saturation indicating that most of microbial diversity was covered.
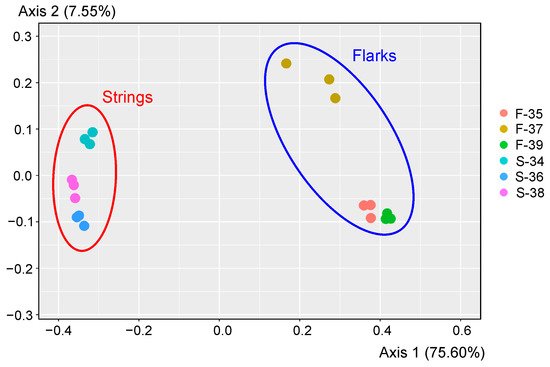
As revealed by the UniFrac analysis, replicate samples clustered together (
Figure 2). Moreover, the microbial communities in different string peat samples were highly similar to each other and were significantly different from those in samples collected from flarks (
p < 0.0001 as revealed by PERMANOVA test) (
Figure 2). Therefore, for subsequent analysis, for each of the peat samples, three replicates were combined into one dataset. Only 445 OTUs were shared between flark and string peat samples further emphasizing the differences between these microbial communities.
Figure 2. Comparison of microbial community composition in analyzed peat samples by principle coordinate analyses (PCoA). PCoA plot is based on weighted UniFrac distance of 16S rRNA sequencing dataset.
The number of species-level OTUs present in individual peat samples ranged between 583 and 1485, these values are typical for peatlands
[33][39]. Alpha diversity indices (
Table 2) indicate that the microbial community composition was more diverse and even in the peat from strings then in the flarks according to the Shannon and Peilous indices.
Table 2. Alpha-diversity metrics.
| Peat Type |
Sample ID |
Richness |
Peilous Evenness |
Jost |
Shannon |
| String |
S-34 |
1937 |
0.803 |
249.7 |
6.08 |
| |
S-36 |
1352 |
0.805 |
199.8 |
5.81 |
| pH ** |
4.62 |
5.19 |
5.04 |
5.5 |
5.94 |
5.74 |
| |
S-38 |
1657 |
0.797 |
218.0 |
5.90 |
T, °C |
18.9 |
17.4 |
16.5 |
13.4 |
17.7 |
| Flark |
F-35 | 15.1 |
| 1230 |
0.706 |
80.8 |
5.03 |
Peat characteristics |
|
|
|
|
|
|
| |
F-37 |
1705 |
0.759 |
152.4 |
5.65 |
Total organic carbon (%) |
97.2 |
98.1 |
97.3 |
97.2 |
97.2 |
97.4 |
| |
F-39 |
1816 |
0.729 |
107.5 |
5.47 |
N-NH4 (mg kg −1) |
171.9 |
141.1 |
155.9 |
182.5 |
329.1 |
237.8 |
| N-NO3 (mg kg −1) |
19.7 |
10.1 |
20.6 |
8.4 |
11.3 |
9.9 |
| SO4 (mg L −1) ** |
259 |
185 |
317 |
52 |
61 |
37,5 |
| Fe (mg kg −1) |
380 |
450 |
460 |
500 |
750 |
830 |
| Ca (mg kg −1) |
8100 |
6500 |
8000 |
3700 |
6000 |
7200 |
| Mg (mg kg −1) ** |
740 |
980 |
1040 |
430 |
570 |
670 |
| P (mg kg −1) ** |
The effective number of species in string samples (Jost index) was nearly twice as high as that in flarks. The differences in Peilous evenness, Jost and Shannon indices between strings and flarks were statistically significant (
p < 0.05).
54. Microbial Community Composition at the Phylum Level
Taxonomic assignment of OTUs revealed the presence of 50 phylum-level bacterial and archaeal lineages defined in the genome-based taxonomy system
[34][40], according to the SILVA v.138 database. However, OTUs representing top 12 phyla of Bacteria and top 4 phyla of Archaea, comprising on average more than 1% of all the 16S rRNA gene sequences in strings and/or flarks, together accumulated more than 92% of the microbiomes (
Figure 3).
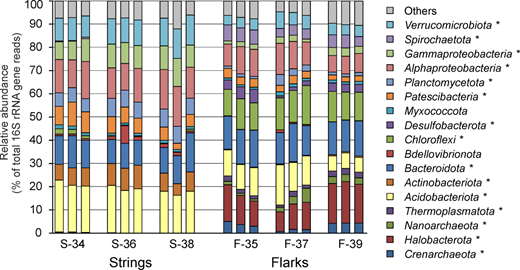
Figure 3. Prokaryotic community composition in string and flark peat samples, according to results of 16S rRNA gene profiling. Community composition is shown at the phylum level, with exception of Proteobacteria, for which classes Alpha- and Gammaproteobacteria are shown. All replicate samples (three per sampling site) are presented. Lineages with statistically significant differences (p < 0.05) in relative abundance in strings and flarks are marked with an asterisk.
The composition of microbial communities of peat from strings and flarks was strikingly different already at the level of domains and phyla (
Figure 3). Archaea accounted for 21.9 ± 2.0% (mean ± standard error, of all 16S rRNA gene sequences) in flarks, but only 0.4 ± 0.1% in string samples. Archaeal populations in flarks were represented by members of the
Halobacterota (13.6 ± 1.1%),
Crenarchaeota (3.2 ± 0.5%),
Nanoarchaeota (2.6 ± 0.5%) and
Thermoplasmatota (1.9 ± 0.2%). Most of Archaea represented known methanogenic lineages, including the genera Methanoregula (10.8 ± 1.1%), Methanocella (1.4 ± 0.2%), Methanosaeta (0.6 ± 0.2%), Methanosarcina (0.3 ± 0.05%), and the family Methanomassiliicoccaceae (1.8 ± 0.2%) of the Thermoplasmatota.
Bacterial communities in strings were dominated by the Proteobacteria (27.8 ± 1.0%), mostly of classes Alpha- (14.3 ± 0.7%) and Gammaproteobacteria (9.6 ± 0.4%). Other abundant groups were the Acidobacteriota (19.2 ± 0.6%), Bacteroidota (12.2 ± 0.7%), Verrucomicrobiota (11.0 ± 0.4%), Actinobacteriota (8.0 ± 0.5%), Planctomycetota (6.8 ± 0.3%), and Patescibacteria (6.8 ± 0.7%). All these lineages were also found in flark samples, but in most cases their relative abundancies were several times lower than in string peats. The exceptions are the Bacteroidota, the dominant phylum in flarks accounting for 14.5 ± 0.4% of the communities, and the Gammaproteobacteria which relative abundance in flarks was about 10%. Three phyla abundant in flarks, Chloroflexi (13.1 ± 0.5%), Spirochaetota (5.2 ± 0.4%) and Desulfobacterota (3.3 ± 0.5%) accounted on average for less than 1% of 16S rRNA gene sequences in the peat string samples. The differences in the relative abundance of all above mentioned phyla between the strings and flarks were statistically significant (p < 0.05).