Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Emil N. Sobol and Version 2 by Camila Xu.
The intervertebral disc (IVD) consists of three main parts: the annulus fibrosus (AF), the nucleus pulposus (NP) and the dense hyaline endplate (EP), which is in the intimate system of the vertebral plate closure.
- laser modification
- tissue structure
- regeneration
- cartilage repair
- spine
1. Introduction. Degenerative Diseases of the Spine. Causes, and Modern Methods of Treatment
1.1. Anatomical, Morphological, and Clinical Concepts of Degenerative Diseases of the Spine
Degenerative diseases of the intervertebral discs (DDD) are currently a serious problem facing the world community [1][2][1,2]. The prevalence of DDD is roughly described in proportion to age, so that 40% of people in their 40 s have DDD, rising to 80% in people aged 80 and over. Each year, 403 million new cases of DDD are registered [3].
The intervertebral disc (IVD) consists of three main parts: the annulus fibrosus (AF), the nucleus pulposus (NP) and the dense hyaline endplate (EP), which is in the intimate system of the vertebral plate closure (Figure 1A). The AF surrounds the NP and consists of dense plates, each of which is formed by longitudinal and parallel collagen fibers (CF), and the direction of these fibers in the adjacent plates is mutually perpendicular. AF consists of fibrous cartilage in which CF are type I and type II collagens. Proteoglycan (PG) complexes—aggreganes—are contained between CFs. Aggreganes are composed of hyaluronic acid and a core protein to which chains of glycosaminoglycans (GAGs) are attached, mainly chondroitin and keratan sulfates [4].
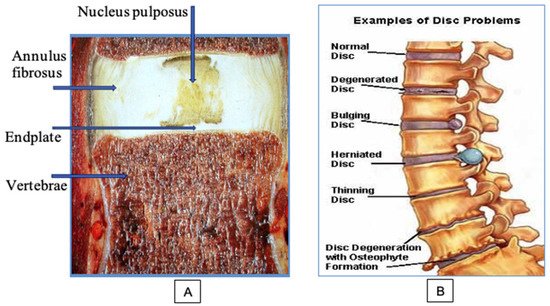
Figure 1. Structure and problems of the spinal disc: (A) main components of the disc; (B) the sequence of the development of degenerative disc diseases.
The NP is the inner part of the IVD; it consists of a highly hydrated tissue of a jelly-like consistency. The basis of the NP is formed by water (80–90%) and large compounds of GAG. The sulfate groups of GAGs carry negative charges that contribute to the maintenance of osmotic pressure in the disc, which ensures the amortization functions of the spine. The amount of cells in the IVD is very low (0.25–0.5%). Cells must maintain the macromolecular structure of the disc, a certain concentration of PG, collagen, proteases, and their inhibitors throughout life. Inside the disc of an adult, blood vessels are completely absent, and nutrition of the cartilaginous structures is carried out from the bodies of the vertebra by diffusion and osmosis through the EP. IVD remains healthy as long as the balance between the synthesis and decay of its macromolecules is maintained.
At the initial stages of development of DDD, the NP is gradually dehydrated. A decrease in pressure inside the disc produced pathological mobility in the vertebral segment. Clinically, this is manifested by the appearance of local moderate pain in the damaged area, a feeling of fatigue, and recurrent attacks of myogenic pain syndrome. Changes appear in MRI. Losing moisture, NP, normally light and homogeneous, gradually fragments and becomes dark in color. This phenomenon is called a dark disc. The progression of the degenerative process in the IVD, dehydration of the NP, and an increase in segment instability led to a significant increase in the load on the AF. As a result, the number of AF microcracks increases; these microcracks gradually transform into functionally significant cracks and ruptures (Figure 1B). Fragments of the NP begin to penetrate these cracks. With sharp physical exertion, the process can be aggravated to a complete rupture of the AF with the loss of sequestered fragments of the destroyed NP into the spinal column.
1.2. Modern Methods of Treatment of Degenerative Disc Diseases
Treatment of DDD is usually based on surgical methods or conservative therapy, including medical pain relief and the use of physiotherapy methods of treatment. Conservative therapy, used for several years, leads to a decrease in pain, but also leads to the appearance of IVD fibrosis. At the same time, the persisting overload of the facet joints of the spine and the phenomena of instability form a new pain syndrome [5]. The use of modern surgical technologies for the treatment of DDD, unfortunately, does not stop the pathological process, but serves as a palliative method that temporarily relieves pain and temporarily improves the patient’s quality of life. Surgical interventions lead to the subsequent decompensation of the process and weaken the compensatory capabilities of the organism itself. Therefore, at present, there is an active search for new methods of treating DDD, and colossal funds are spent on research in this area [6]. Thermal inactivating pain receptors in IVD is carried out by percutaneous radiofrequency (RF) thermo-coagulation or intradiscal electro thermotherapy (IDET) [7][8][9][10][7,8,9,10]. RF and IDET methods do not stimulate biological disc remodeling and repair. After some time, the pain syndrome progresses again [11][12][13][11,12,13]. Various methods of nucleotomy have become another area of intradiscal decompression [14]. Some authors suggested that fenestration and partial removal of IVD material would reduce intradiscal pressure and nerve root irritation. For this, both endoscopic and puncture access were used, with various types of nucleotomes under X-ray control. Despite the low invasiveness, cost and comparative safety of puncture techniques, the problem of relapses, postoperative instability, and low efficiency (60–70%) of such operations remains. During long-term follow-up from 5 to 25 years for patients after open classical discectomy, based on radiological changes, acceleration of disc degeneration was noted in 48.7% of cases. Microsurgical technique reduces this risk, provoking negative changes in 9.1% [15]. One of the problems, especially in the case of operations aimed at removing a hernia, is a recurrent herniated disc, which can reach 10–15% of operated cases [16][17][16,17]. The implants used to close the defects of the AF designed to reduce the number of relapses did not confirm their effectiveness in the long-term period after surgery [18].
At the beginning of the 21st century, fusion and implantation operations became widespread [19]. However, there are randomized studies that show that the placement of implants increases the risk of undesirable effects (nerve damage, blood loss, inflammatory and general complications), while increasing the time of surgery and the risk of revision spine surgeries [20][21][20,21].
In recent years, attempts to counteract degenerative disc damage is also associated with biological methods with intradiscal stem cell transplantation. Despite some laboratory advances, the clinical use of stem cell transplantation has led to mixed results. In particular, after transplantation of one’s own mesenchymal stem cells into degeneratively affected IVDs with characteristic pain syndrome in provocative discography, after a year, none of the patients showed a decrease in the intensity of back pain [22]. At the same time, some researchers have found beneficial effects in reducing pain and improving disc morphology [23][24][23,24]. Four to six years after such procedures, their safety is reported; however, disc changes were not of a unidirectional, favorable nature, as they combined both positive and negative signs of the progression of the degenerative process [25].
1.3. Lasers in Spine Surgery
Many studies on the use of lasers in orthopedics refers to ablative destructive surgery of the spine. For the first time, percutaneous laser discectomy (PLD) was performed in 1987 using a Nd: YAG laser [26]. With the development of the PLD technique, its clinical success increased to 80% or more [27]. It was found that in the tissues surrounding the ablation zone significant damage is possible due to heating. This can cause complications, often including an infectious lesion of the operated IVD—discitis in the zone of tissue necrosis after laser evaporation of the central part of the NP. There is aseptic discitis, root damage, thermal damage to the EPs of adjacent vertebrae. One year after PLD, necrotic tissues are found in the disc. A decrease in the likelihood of side effects has been achieved through selective ablation of the NP and by reducing the radiation power. Transforaminal epiduroscopic laser ablation (TELA) selectively removed the herniated portion of IVD and induced more effective decompression with minimal complications [28]. TELA enhances the resorption of the herniated IVD by drilling a hole in the AF and partially removing the slipped NP with the use of a 1414 nm Nd:YAG laser. Although the clinical success rate was 76.6% at 12 months, it is unclear whether a better outcome can be achieved with direct AF ablation.
In [29], a technique for laser irradiation of an IVD with a moderate power—thermodiscoplasty (TDP)—is presented, which is based on the effect of collagen compression when heated above 70 °C. TDP leads to tissue compaction and reduction in its volume without carbonization or significant destruction. The TDP method is used during endoscopic removal of a hernia or disc protrusion, when using an endoscope, it is possible to accurately bring the laser probe into the area of the pain generator, including after mechanical removal of the restrained fragments of the NP. At the same time, in the zone of laser exposure, there was histologically a thickening and homogenization of CFs as well as destruction of most of the cells. Laser radiation made it possible to vaporize the NP of the disc, while simultaneously producing its de-reception. The success rate was 95.2%, and in many cases, the postoperative control MRI showed a decrease in protrusion size. Even though the total energy of laser action on the IVD was significantly lower (2–3 times) than with the accepted puncture laser discectomy, this action nevertheless caused extensive tissue coagulation and, consequently, the destruction of cellular elements. This, unfortunately, significantly worsened the dynamics of reparative processes in the IVD.
A fundamentally new approach to the treatment of DDD is based on laser-induced modification of the IVD tissues [30]. Moderate heating and control of thermomechanical stresses in tissues subjected to short-term non-destructive pulse repetitive laser irradiation make it possible to form new cartilaginous tissue of the fibrous-hyaline and hyaline types in the IVD [31]. Activation of reparative regeneration was observed as early as on the 7th day, and replacement of AF and NP defects with new cartilaginous tissue occurred 2–3 months after laser exposure. The developed technology of laser reconstruction of discs (LRD) makes it possible to treat IVD by replacing disc disrupture and disc fissures with regenerating cartilaginous tissue [32][33][32,33]. This contributes to the reverse development of the degenerative-dystrophic process with stabilization of the dorsal segment due to the replacement of altered IVD tissues with regenerating hyaline and fibro hyaline cartilage [30][31][32][33][30,31,32,33]. In addition, the formation of a new dense cartilage limits the migration of NP fragments, preventing the recurrence of disc herniation [34]. This is due to the transformation of the NP into hyaline cartilage, which tightly grows together with neighboring tissues and without clear boundaries passes into AF and hyaline EP. The thermal effect of laser radiation leads to de-reception of the disc and the effect of “compression”, which ensures a decrease in the size of the protrusion. Moreover, the replacement of degeneratively destroyed IVD areas with new regenerating cartilaginous tissue limits the expansion of the innervation zone in the disc and thereby eliminates the development of discogenic reflex pain syndromes [33][34][35][33,34,35].
Currently, among neurosurgeons and orthopedists, there are two opposing points of view on the possibilities, advantages, and disadvantages of ablative laser technology for the treatment of IVD. Along with a critical but generally positive assessment of the results of laser surgeries, the technology of which goes through various stages of development, a skeptical assessment of the prospects for the use of ablative lasers in spinal surgery has received a certain spread. The authors of [36] write: “Despite the fact that lasers have long been used in pain treatment procedures such as percutaneous discectomy, the alleged benefits of lasers, such as reducing inflammation and degeneration, have not been supported by reliable clinical studies.” On the other hand, the authors of [37] note that after a 2-year follow-up, the strategy of laser percutaneous disk decompression by results is not inferior to microdiscetomy and, as a minimally invasive method, can take its place in the arsenal of treatment for radiculitis caused by herniated IVD. An example of a negative assessment of ablative laser surgery is the work [38], which notes that several randomized clinical trials have shown the lack of clinical efficacy of PLD-type procedures compared to microdiscectomy. It is noted that laser procedures are often performed by pain specialists without special surgical experience who are unable to eliminate the postoperative complications of PLD. There are doubts about the feasibility of using PLD for the treatment of minimal disc herniation, since such patient problems often resolve spontaneously within a few months without surgery. In a recent review article on the use of lasers in neurosurgery [39], the following conclusion was formulated: “The many ways in which lasers are used in neurosurgery are evidence of the technological advances and practicality of laser science. Despite the difference of opinion, the use of lasers for minimally invasive procedures shows promising results and deserves further study.”
It should be emphasized that the above doubts relate mainly to destructive operations on the IVD using ablative laser radiation. The authors of negative statements did not consider the new restorative technology of LRD, which eliminates the adverse consequences associated with damage to surrounding tissues [31][32][33][34][35][31,32,33,34,35]. In contrast to ablation technologies, the use of moderate radiation powers in LRD allows non-destructive modification of the tissue microstructure. For the processes of laser modification, the correct choice of parameters is especially important, which makes it possible to control the ongoing physicochemical and biological processes by controlling the temperature and thermomechanical stresses in the laser treated zone. This makes it possible to obtain a stable positive effect of LRD [33][35][33,35].
2. Non-Destructive Laser Modification of Cartilage Is the Basis of the New LRD Approach
2.1. Physicochemical and Biological Processes in the Disc Tissues under Laser Radiation
When exposed to laser radiation on disc tissue, various processes occur, including optical (absorption and scattering of light), thermal (heating and cooling), mechanical (the formation and propagation of mechanical stresses, changes in the strength characteristics of the tissues), electrical (redistribution of charges, change in electrical conductivity) and physicochemical processes (rupture of chemical bonds, phase transitions and chemical reactions, formation of gas bubbles, denaturation, coagulation, tissue carbonization). At the same time, various biological processes (proliferation and death of cells, transformation of the cartilage matrix, and tissue regeneration) also occur, which can lead to positive or negative medical effects.
Laser-induced phase transformations and chemical reactions in tissues occur in several stages. The first stage of laser modification of the structure and mechanical properties of cartilage is a change in the state and structure of interstitial water. Depending on the power and duration of laser heating, the subsequent stages of laser exposure can be various processes, such as structural changes in the collagen and proteoglycan subsystems, local mineralization, local melting, and the formation of micropores in the cartilage matrix [40]. The study of collagen stability in AF was carried out by the method of differential scanning calorimetry [41]. It was shown that the violation of the structural organization of the IVD collagen network is determined not only by the maximal temperature of the heated zone, but also by the space–time temperature distribution. Short-term (within 5–10 s) heating of IVD tissues to temperatures of 65–70 °C does not lead to a noticeable denaturation of CF. At the same time, the non-uniform temperature distribution leads to the expansion of heated zones, the movement of interstitial water and the development of thermal stresses in the affected area. As a result, local foci of violation of the strictly ordered arrangement of CFs and fibrils arise. If the temperature of the tissue in this case turns out to be above 80 °C, then a noticeable denaturation of collagen occurs, which leads to local shrinkage of the CFs. This process, in turn, stimulates the development of additional tension on adjacent fibers, which can lead to rupture of individual CFs]. Thermomechanical stresses affect cells, the structure and thermal stability of the extracellular matrix (ECM), both in the heat-affected zone and outside it [41]. The results of these experiments can explain the reasons why various currently used physical methods associated with significant heating of AF (including laser, electrothermal, or RF) lead to a decrease in the mechanical and thermal stability of IVD tissues, which, in a number of cases, is the cause of postoperative complications when exposed to intense energy sources with excessively increased or poorly controlled power. Therefore, the main target of laser action in the LRD technology was chosen to be the NP of the disc and the EP region without a significant effect on the AF [32].
2.2. Goals, Objectives, and Targets of Laser Treatment
Targets for laser effects and possible types of cartilage reactions to laser radiation were discussed in detail in [34][35][42][34,35,42] and are shown in Figure 2.
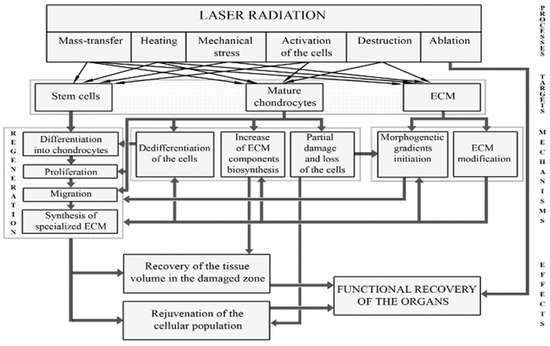
Figure 2.
Targets for laser radiation and regeneration processes leading to the restoration of IVD.
Laser radiation can directly affect (a) cells; (b) various components of the ECM; (c) signaling molecules produced by cells; (d) intercellular interactions; (e) differentiation and dedifferentiation of cells, their migration and biosynthetic activity. Possible processes and pathways for cartilage regeneration include: (1) additional supply of cells; (2) enhancement of biosynthesis of ECM components; (3) stimulation of mature chondrocytes; and (4) activation of resident stem cells in the direction of their proliferation, differentiation, and production of ECM. Unlike ablative or low-energy laser treatment, modifying laser radiation causes controlled thermal and mechanical effects (both on cells and on ECM), which leads to activation of tissue regeneration. Spatio-temporal modulation of laser radiation allows to control the actual distribution of stretched and compressed zones in the cartilage.
Mechanical loads are important factors governing the chondrogenesis orchestra, including the processes of cell differentiation [43]. Thermomechanical laser action can play a decisive role in the differentiation of immature cartilage cells. The advantage of laser action on the proliferation of chondrocytes in comparison with other thermal, mechanical, and chemical effects was demonstrated in [44]. Laser radiation can stimulate the processes of proliferation and the acquisition of a specialized phenotype by resident or mesenchymal stem cells, promoting their transformation into mature hyaline-like chondrocytes. It is important that laser modification of the fine structure of the ECM does not change its overall organization. This provides a natural environment for chondrocytes and leads to the restoration of hyaline-type cartilage [32][42][32,42].
2.3. Mechanisms of Laser Regeneration
Laser activation of cell regeneration can occur as a result of the direct action of laser radiation on cells, and indirectly—through the ECM due to the modification of its structure and the formation of temperature and mechanical stress fields. It is known that chondrocytes are sensitive to environmental conditions, in particular, to temperature and mechanical stress [45][46][45,46]. Modulated in space and time, laser radiation causes pulsed-periodic heating, leading to inhomogeneous thermal expansion and inhomogeneous pulsating field of mechanical stresses, which can actively affect the function of chondrocytes, contributing to their proliferation and biosynthetic activity. Compressive stress of a certain frequency (0.1–10 Hz) and amplitude (5–25 MPa) promotes the proliferation of chondrocytes and the activation of the synthetic activity of cells to produce collagen and proteoglycans [47][48][47,48]. Going beyond the indicated boundaries of the vibration frequency range and an excessive increase in the amplitude of mechanical action leads to inhibition of the life of cells and to their apoptosis [48].
Mechanical forces act on processes in the cells through mechanotransducers PIEZO1 and PIEZO2, which convert external mechanical stresses into electrical signals that control cell metabolism and apoptosis [49][50][49,50]. Another important mechanism of laser regeneration is the formation and distribution of thermo-shock proteins, cytokines, and growth factors, released by a small fraction of cells dying as a result of laser action [51]. According to the data [52][53][52,53] obtained in experiments with cultures of chondrocytes, growth factors and cytokines increase the production of proteoglycans and type II collagen, cause accelerated cell proliferation, and inhibit their apoptotic death. In experiments [54], the induction of chondrogenic differentiation of bone marrow stem cells under the influence of growth factors and glucocorticoids were found. In vivo studies, an increase in IVD mass and proteoglycan content and the appearance of cell clusters similar to clusters of normal hyaline cartilage were found [55].
The third important mechanism of laser regeneration is the modification of the porous structure of the ECM (Figure 3). Loosening of the matrix is observed using an optical or ultrasound microscope [40][56][40,56], and a more precise microscopy (atomic force microscope, structured irradiation microscope, and electron microscope) makes it possible to study specific pores of micron and submicron size [57]. Micropores increase the diffusion of water and nutrients in the cartilage matrix and thus promote regeneration. In this case, there are no macroscopic structural changes and deterioration of the mechanical properties of the cartilaginous tissue [32][40][32,40]. The pore size in ECM is of great importance. Macroscopic pores and defects (ranging in size from hundreds of microns to several millimeters) tend to become overgrown with cells and matrix. Small pores a few microns in size, as a rule, do not overgrow. Such micropores, formed in the cartilaginous tissues of the IVD and joints of animals as a result of laser irradiation, were observed several months after laser exposure [42][57][42,57].
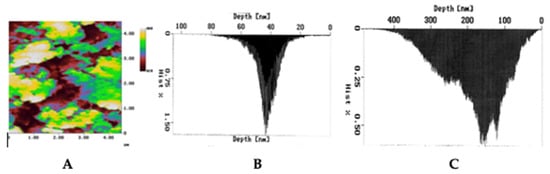
Figure 3. Formation of micropores in the rabbit cartilage plate under laser action studied by Atomic Force Microscopy: (A) image of micropores; size distribution of micropores (B) before and (C) after laser exposure.
The regeneration processes discussed above are associated with a slight heating of the tissue and can be called thermomechanical mechanisms. Regarding the mechanisms of photobiomodulation, low-level laser therapy (LLLT) does not lead to noticeable heating of the tissue. Various hypotheses have been put forward concerning various chromophores [58][59][60][61][62][58,59,60,61,62]. The effects of laser radiation over living tissues are based on the absorption of its energy and its transduction into a biological process, mainly due to Adenosine triphosphate (ATP) synthesis. LLLT may modulate transcription of several growth factors, including fibroblast growth factors (FGF) and vascular endothelial growth factors (VEGF) [63]. There is evidence that both ATP activation and cell proliferation under the influence of R-NIR light occur through the interaction of photons with intracellular water layers. The interaction has at least two biologically important effects: a change in density (volumetric expansion) and a decrease in the viscosity of water [64]. LLLT can increase proliferation of the marrow stem cells without producing high levels of reactive oxygen species [65]. Bone marrow irradiated in vivo can be used to treat a variety of disease conditions. The irradiation may be performed by transcutaneous application of infrared laser radiation over the area of a marrow-containing bone. It was found that labeled cells were seen in and near the infarct up to eight weeks post myocardial infarction, while none were seen in sham-operated hearts. It was concluded that following myocardial infarction, mesenchymal stem cells are signaled and recruited to the injured heart, where they undergo differentiation and may participate in the remodeling process [66].
A detailed analysis of photomodulation processes is beyond the scope of this work. Here, we just note that a comparative study carried out in [47] showed that the thermomechanical effect of the laser radiation causes a more pronounced stimulation of chondrocytes than photomodulation. A recent review of publications on the use of lasers in the treatment of pain syndrome of the musculoskeletal system also showed certain advantages of moderate radiation power compared to low-intensity laser exposure [67]. The conclusion made in [68] seems to be important that “Treatment of pain syndrome of the IVD is possible without complete recovery of age-related and degenerative changes in the NP by accelerating the healing of damage to the periphery of the disc by stimulating cells, accelerating the transport of metabolites and preventing adhesions and repeated injuries”. This approach corresponds to the LRD technology, which not only reduces pain, but leads to the restoration of cartilage of IVD with partial replacement of the NP tissue with hyaline cartilage. Laser radiation modulated in space and time allows for precise control of various parameters important for the process of structure modification and regeneration, i.e., temperature, amplitude, and frequency of mechanical action, as well as mass transfer into cells, which leads to the appearance of morphogenetic gradients and control of tissue regeneration.
