Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Rita Noelle Moussa and Version 2 by Camila Xu.
Anaerobic digestion (AD) is an established process used across the world for the conversion of biomass and organic waste into methane, a renewable energy vector.
- hydrogen
- dark fermentation
- organic waste
1. Introduction
Anaerobic digestion (AD) is an established process used across the world for the conversion of biomass and organic waste into methane, a renewable energy vector [1][2][3][1,2,3]. While the use of AD to produce methane is an established commercial process, to the best of our knowledge, there are, to date, no full-scale processes in which AD, also called dark fermentation (DF) in this case, is used for the primary purpose to produce the intermediates of the process, i.e., hydrogen and short-chain organic acids (SCOAs). This is despite the large volume of recent research, at lab or pilot scale, on DF to produce these intermediates [4].
Anaerobic digestion consists of several steps. Hydrolysis of polymeric biomass components, e.g., proteins, to their monomers is the first step and is often rate limiting [4]. During the second step, also called acidogenesis, various compounds (hydrogen, SCOAs, alcohols) are produced as intermediates. Generally, in conventional AD, these compounds are converted to methane in the final step of methanogenesis. However, the intermediates produced in the second step can be more economically valuable than the end product methane [5].
Hydrogen gas is considered a replacement for fossil fuels in the future because of its environmental and socio-economic advantages [6]. To date, hydrogen has been produced at a commercial scale at global rates, in the order of 50 million tons per year, and is mainly used in chemical synthesis, e.g., for ammonia and methanol synthesis and hydrogenation reactions. There is increasing interest in the use of hydrogen as a renewable energy vector when produced from water electrolysis using renewable electricity. However, currently, most of the hydrogen produced comes from natural gas, which is a non-renewable, therefore non-sustainable, fossil resource, at least in the medium-to-long term [7].
The production of hydrogen from DF of biomass or organic waste has several environmental advantages, compared with its production from natural gas or from water electrolysis. Compared with the use of natural gas, DF uses renewable resources or, even better, wastes that need to be treated. To produce hydrogen, the DF of organic waste can represent the first stage of the waste treatment and valorisation process. In addition, and in contrast with the chemical synthesis of hydrogen from natural gas (steam methane reforming), DF uses mild temperatures close to ambient values and does not need the external addition of metal catalysts, with clear environmental benefits. Compared with the use of water electrolysis, DF is less energy intensive. Considering several renewable energy scenarios for the UK, our recent study has shown that energy from organic waste, including hydrogen production from DF, could potentially provide up to ~30% of the heating and transport energy in this country [8].
However, despite the potential environmental benefits, hydrogen production from DF of biomass and organic waste is still limited at the lab or pilot scale [9]. In order to have a successful commercial process, it is necessary to maximise the hydrogen yield, productivity, and content in the fermentation gas. High yield is important to maximise the hydrogen produced from a given mass of biomass/waste. High volumetric productivity corresponds to a high hydrogen production rate per unit volume of the reactor and is therefore essential to minimise the volume of the equipment and the capital costs of the process. Hydrogen content in the fermentation gas should also be maximised to minimise the separation costs due to the removal of other components, usually mainly carbon dioxide.
Many process parameters can affect hydrogen yield, productivity, and content. The feedstock concentration is expected to affect hydrogen productivity while potentially also affecting the yield because of inhibition effects. The residence time can affect the extent of biomass degradation and the possible conversion of hydrogen into methane by methanogenic microorganisms. The residence time also can directly affect the process productivity. As with any other biological process, DF can be affected by pH and temperature.
2. Comparison with Other Studies in the Literature
Various studies have investigated the effect of different operating parameters on hydrogen production, focusing on one or two operating parameters. The results were not widely reproducible due to the range of operating conditions, the nature of the inoculum, and the substrate used. Therefore, the importance of our study is to combine and analyse the results of previous studies to identify general trends and optimal factors affecting hydrogen production.
Previous studies have highlighted the importance of pH on the performance of dark fermentation and hydrogen production. The pH can affect the metabolic pathway of fermentative bacteria, especially hydrogen-producing bacteria, and influence hydrogenase activity [10][11][69,70]. While fermentation of organic compounds occurs at a pH range between 4 and 9, hydrogen-consuming bacteria or methanogens have an optimal pH range between 6.8 and 8 [12][13][71,72]. This can explain the finding of our analysis which indicates that acidic pH is the most favourable for hydrogen production with dark fermentation.
Regarding the temperature, most of the studies in the literature on dark fermentation have used mesophilic conditions. Thermophilic conditions have many disadvantages over microbial growth which will negatively affect hydrogen production. The increase in temperature involves cellular growth inhibition, thermal denaturation of proteins especially enzymes, decrease in extracellular polymeric substances, and most of all increase in energy cost [14][73]. However, our analysis shows that, although there is a rather weak positive effect of mesophilic conditions, hydrogen production with high yield is also possible under thermophilic conditions, e.g., high hydrogen yields (20 and 21% COD COD−1) were obtained at 70 and 60 °C, respectively, under neutral pH (Figure 1, Figure 2 and Figure 3; parts e,f).
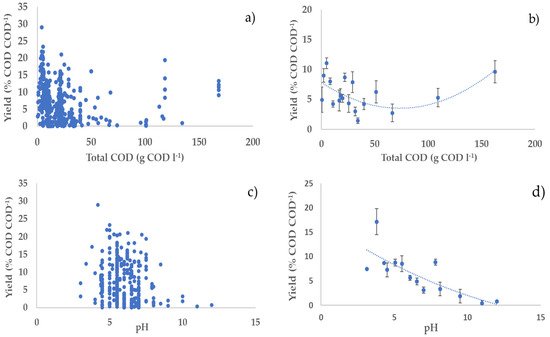
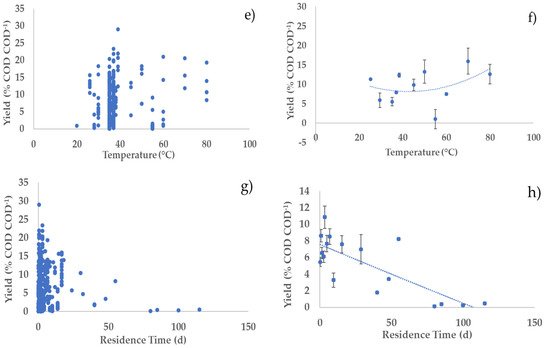
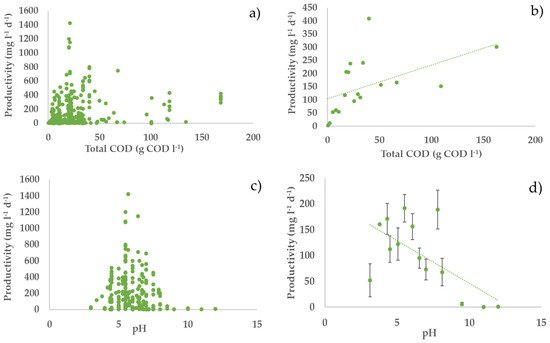
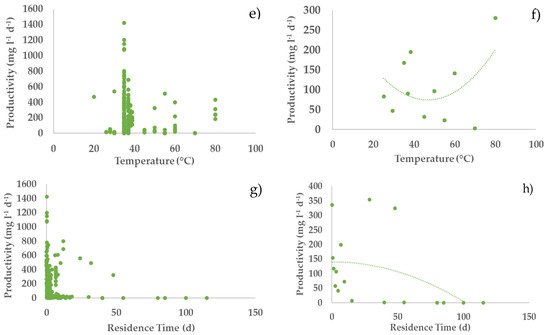
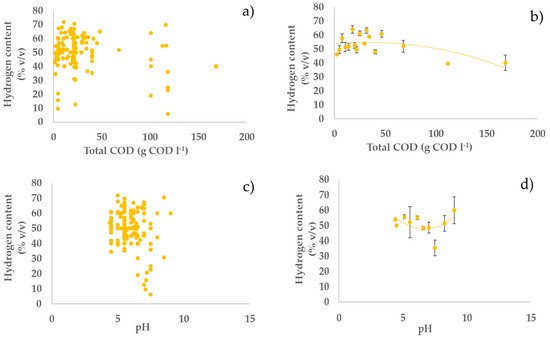
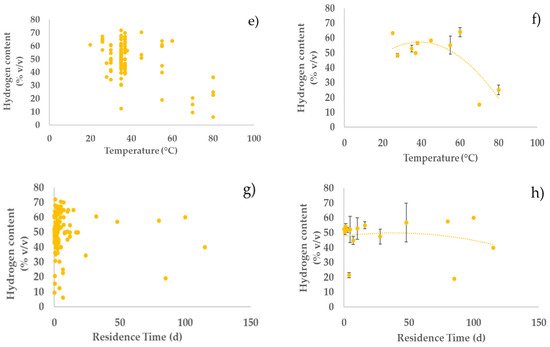


Figure 1. Relationships between hydrogen yield and operational parameters: (a,c,e,g) all considered experiments; (b,d,f,h) average values of experiments in each narrow range (width maximum 20%) of the operating parameters (error bars indicate the standard error) with second-order polynomial curve. The polynomial curve is only used to visualise the general trend, without any statistical significance.


Figure 2. Relationships between hydrogen productivity and operational parameters: (a,c,e,g) all considered experiments; (b,d,f,h) average values of experiments in each narrow range (width maximum 20%) of the operating parameters (error bars indicate the standard error) with second-order polynomial curve. The polynomial curve is only used to visualise the general trend, without any statistical significance.


Figure 3. Relationships between hydrogen content in biogas and operating parameters: (a,c,e,g) all the considered experiments; (b,d,f,h) average values of experiments in each narrow range (width maximum 20%) of the operating parameters (error bars indicate the standard error) with second-order polynomial curve. The polynomial curve is only used to visualise the general trend, without any statistical significance.
Concerning the residence time, Liu et al. observed a decrease in gas (hydrogen and methane) production during AD of organic household solid waste, by thermophilic microorganisms at 70 °C, when the residence time was decreased from 6 d to 1 d [15][74]. Furthermore, hydrogen production yields were higher at shorter HRT (2 h), in continuous mode using a concentrated substrate, slightly acidic pH, and high temperature (55 °C) [16][13]. Similarly, an increase in hydrogen production rate was observed when HRT decreased from 8 h to 1 h [17][75]. An optimal HRT of 2 h resulted in the highest hydrogen production during the AD of cassava wastewater in an anaerobic fluidised bed reactor at 28 °C. On the other hand, Ziara et al. achieved the highest hydrogen yields (0.85 mol H2/mol lactate) by fermenting lactate wastewater at 45 °C for 180 h, with an initial pH of 8.5 [18][39]. Additionally, this study confirms the impact of short residence time on hydrogen yields, productivity, and content (Figure 1, Figure 2 and Figure 3; parts g,h). However, one experiment conducted by Yu et al. noted an increase in hydrogen yield, from 1.74 mol H2/mol hexose to 2.14 mol H2/mol hexose, when HRT increased from 2 h to 24 h [16][13]. The outcomes depend on the nature of substrate, its solubility, availability, and on the nature of the inoculum. To further improve hydrogen yields from the AD of organic waste and wastewaters, it is important to take into consideration all the parameters discussed above as complementary and not individual parameters.
3. Process Considerations
Although the performance of a hydrogen production process with DF will be highly dependent on the specific feedstock used, this study offers some general insight on how to optimise process parameters to maximise process performance. Generally, higher substrate concentration in the feedstock should be preferred, as it tends to give higher hydrogen productivities with a relatively small impact on hydrogen yield and content in the biogas. Acidic pH values should be preferred over neutral and alkaline values. Indeed, acidic pH values generally tend to give higher yields, with relatively little, but still positive effects on hydrogen productivity and content. pH control to acidic values seems an interesting strategy to maximise hydrogen production by reducing the conversion of hydrogen into methane. It is interesting to observe that DF naturally tends to be acidic due to the production of SCOAs; therefore, it is likely that an acidic pH can be maintained without the addition of pH balancing chemicals, which increases the attractiveness and environmental sustainability of this process. As far as the temperature is concerned, our analysis indicates that hydrogen production using AD is relatively unaffected by temperature in a wide range, 30–60 °C, with some indication of a slightly better performance at the lowest end of this range. From the process point of view, this indicates that mesophilic conditions should generally be preferred to thermophilic conditions, because of the lower energy consumption to maintain the temperature of the digester. Our analysis indicates that residence time should be maintained at relatively low values to maximise hydrogen production. Indeed, low residence time contributes to washing out the methanogens, which, in turn, contributes to increasing hydrogen yield. Notably, low residence time means smaller volumes of the reactors, which has a direct positive impact on the volumetric productivity, as observed in our analysis. Furthermore, smaller reactors also contribute to decreasing the capital and operating costs of the process.
Another process variable that tends to favour hydrogen production is the nature of the feedstock, with soluble substrates tending to give higher yield and productivity than insoluble substrates. Overall, the pretreatment of the substrate and the inhibition of methanogenesis seem to have only a modest effect on the process. It is interesting to observe that continuous fermentation seems to have a relatively large positive effect on productivity.
The most favourable conditions for hydrogen production identified in this analysis—namely, high substrate concentration, acidic pH, and short residence time—should be targets of more research effort to optimise the process for full-scale commercialisation. Clearly, the economics and sustainability of the process should also be investigated. On the other hand, it is worth noting that only less than 3% of the considered studies investigated hydrogen production from municipal wastewaters. Although municipal wastewaters are typically diluted, their conventional aerobic treatment is energy intensive, and an anaerobic process with hydrogen production in the first stage and methane production in the second stage would represent an interesting alternative to current processes. This also deserves further investigation.
