Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Azizur Rahman and Version 2 by Jessie Wu.
Marine organisms harbor numerous bioactive substances that can be utilized in the pharmaceutical and cosmetic industries. Scientific research on various applications of collagen extracted from these organisms has become increasingly prevalent. Marine collagen can be used as a biomaterial because it is water-soluble, metabolically compatible, and highly accessible.
- marine collagen
- skin
- jellyfish
- wound healing
- bone regeneration
- antiaging
1. Introduction
Marine organisms such as fish, jellyfish, sponges, and other invertebrates harbor a significant source of collagen and are highly advantageous over other sources, as they are metabolically compatible, lack religious constraints and are free of animal pathogens [1][2][3][4]. In fact, fish skins are commonly used for type I collagen extraction, as they are not only immensely abundant but also do not have religious restrictions and are not a risk of disease transmission [5][6][7]. Land animals possess many transmittable diseases, which makes them less favorable for use in industries. For example, cattle, although a large source of collagen, pose risks for bovine spongiform encephalopathy (BSE) as well as transmissible spongiform encephalopathy (TSE) [3][8][9]. These progressive neurological disorders affect cattle and can result in life-threatening infections in humans [3]. In addition, some religious constraints on the use of bovines for the pharmaceutical and cosmetic industries are up for debate [9]. These factors make marine sources of collagen a much safer, easier, and promising alternative.
Skin wounds may take a long time to heal and often do not heal completely. Marine collagen isolated from organisms like fish, jellyfish, and sponges has been implicated in several studies on its potential for increasing wound healing rates [10][11][12][13][14][15]. The processes involve increased fibroblast and keratinocyte migration as well as vascularization and epidermal growth [16][17][18]. In addition to accelerating wound healing, marine collagen has also been shown to have anti-aging properties by slowing the aging process in mice [19][20][21][22]. Studies on humans have also shown that marine collagen can reduce wrinkles, improve skin elasticity, and enhance the overall structure and appearance of skin. Furthermore, collagen’s ability to regenerate bone has been shown to be successful in rat models of menopausal osteoporosis [23]. Marine collagen is able to increase bone mineral density and osteoblastic activity, serving protective effects against bone degeneration [23][24][25][26][27]. Collagen has also been shown to induce chondrogenic differentiation and prevent the development of osteoarthritis (OA) [28][29].
2. The Potential Role of Collagen in Bone and Cartilage Regeneration
Marine collagen sources serve not only as a promising avenue for healing skin injuries but also for bone-related trauma and regeneration. Bone fracture repair and healing is a form of tissue regeneration and is a complex process involving bone formation and breakdown [30][31]. Often, patients present with conditions that require reconstruction of large bones as a result of genetic abnormalities, trauma, infection, and tumors [32]. There is an increasing demand to improve methods of bone repair and regeneration, such as functional bone grafts [33].
Marine collagen bioactive peptides are known to aid in the absorption of calcium and zinc, which are important components of bone and are beneficial for osteoporosis prevention [34][35]. A study performed by Xu et al found that marine collagen peptides isolated and derived by hydrolysis from chum salmon increased serum osteocalcin in treated rats compared to controls. Osteocalcin is a protein hormone secreted by osteoblasts and plays a role in bone maintenance and regeneration through interaction with calcium. The study also found that bone organic matrix, density, femoral length, and femur mineral ions were significantly higher in the collagen-treated group than in the controls [34]. It was hypothesized that the increase in bone mineral density was likely due to increased osteoblast activity, as seen by the increase in bone size and serum osteocalcin [34]. These results shed light on the potential collagen peptides involved in mineral deposition, bone matrix development and an increase in osteoblastic activity, which strongly suggests that collagen is a promising biomaterial for the prevention and treatment of osteoporosis [34]. Osteoporosis and net bone loss are prevalent among aging women going through menopause resulting from estrogen deficiency [23]. Nomura et al. demonstrated that 20 mg of collagen isolated from shark gelatin also increased the bone mineral density of the spongy bone in rat models of menopausal osteoporosis [23].
Furthermore, the biological effect of marine collagen on rat-derived bone marrow stem cells has also been demonstrated. Liu et al. showed that 0.2 mg/mL collagen isolated from fish promoted cell survival and upregulated the expression of several osteogenic and endothelial markers [24]. As shown in Figure 1, there was a significant increase in cell viability at 0.2 and 0.02 mg/mL in the collagen-treated groups [24]. Interestingly, the 2 mg/mL-treated group showed no significant differences due to the high dose resulting in a complex negative feedback mechanism that suppressed cell proliferation [24].
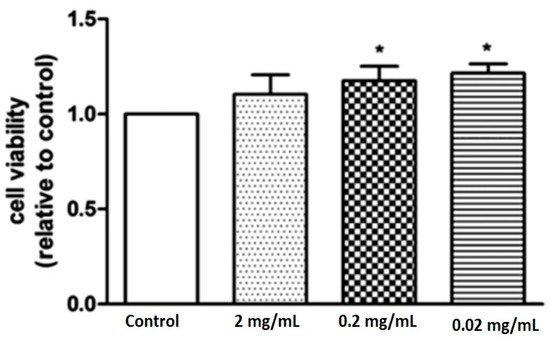
Figure 1. Figure shows the cell viability of rat-derived bone marrow mesenchymal stem cells in the control and marine collagen-treated groups [24]. The results show a significant increase in cell viability at 0.2 and 0.02 mg/mL in the collagen-treated groups (marked with *). The 2 mg/mL-treated group revealed no significant differences due to the high dose resulting in a complex negative feedback mechanism that suppressed cell proliferation.
In addition, osteogenic markers, such as alkaline phosphatase (which enhances the differentiation of cells into osteoblast/bone-forming cells), were significantly upregulated in the collagen-treated groups at 3 and 10 days post-treatment [24]. Similar to this study, Elango et al. found that collagen-treated bone marrow stem cells and mature osteoblastic cells depicted dose-dependent increased proliferation compared to controls [26]. Additionally, osteogenic marker mRNA and protein expression significantly increased in the treated groups compared to controls [26]. These results suggest that collagen is able to promote stem cell differentiation and osteoblastic activity. Yamada et al. also showed that marine collagen peptides extracted from both bone and skin of fish were able to increase osteoblastic cell proliferation, expression of osteogenic markers and mineral deposition [36].
In addition to the use of hydrolyzed collagen peptides, collagen scaffold structures have been shown to be beneficial with regards to bone regeneration. Diogo et al. found that collagen-calcium phosphate scaffold structures crosslinked with EDC/NHS supported the attachment and production of bone-building cells [37]. A more recent study found that within the jellyfish collagen scaffolds, there was greater de novo bone formation and increased macrophage recruitment compared to control groups [38]. Inflammatory cells, such as macrophages, are known to promote tissue repair, regulate inflammation and homeostasis, which is promising for bone tissue regeneration [38]. Similar to the above studies, Rachmawati et al. found that collagen scaffolds isolated from the Aurelia aurita jellyfish helped regenerate alveolar bone [39]. When treated with the collagen scaffold, there was increased osteoblasts and decreased osteoclasts compared to the control group suggesting a potential for alveolar bone regeneration. STRO-1, a biomarker for mesenchymal stem cells and osteocalcin, a protein hormone synthesized by osteoblasts were also increased in the collagen treated groups. These results suggest promising bone regeneration properties [39].
Marine sponges, also known as poriferans, serve as an important source of collagen and have a structure that resembles the cancellous architecture of bone tissue. Lin et al. conducted an in vitro assay using fibrinous collagen isolated from the Callyspongiidae marine sponge [40]. The study observed osteoblasts were able to anchor onto the surface of sponge fibers, proliferate, and grow on the cell-sponge constructs. The study also assessed the osteoconductive potential of the sponge collagen scaffold constructs and found that after 7 days the gene expression of two osteogenic markers, osteocalcin, and osteopontin, significantly increased [40]. On day 14, alkaline phosphatase gene expression, an indicator of osteoblastic differentiation, also significantly increased [40]. Similarly, Green et al. also utilized a marine sponge skeleton scaffold to assess whether collagen could induce osteogenesis [41]. The study found that human osteoprogenitor cells were able to attach to the scaffold within 16 h, and by 21 days, osteoprogenitor cells secreted an extracellular matrix. Furthermore, at 9 and 14 days, alkaline phosphatase activity significantly increased compared to controls [41]. All together, these results indicate that collagen fibers in the marine sponge skeleton provide a scaffold framework for osteoblast attachment, proliferation, and migration, which suggest a promising potential for use in bone tissue engineering [40][41].
The biomedical applications of marine collagen are not limited to skin and bone but also encompass cartilage regeneration. Osteoarthritis (OA) is characterized by a disturbance in cartilage homeostasis, which lacks self-repair and regenerative potential [42][43]. In OA, degradation of the cartilage occurs, which results in exposure of the subchondral bone, and this negatively impacts ones quality of life due to painful and stiff joints [43]. Promisingly, marine collagen has been shown to induce chondrogenic differentiation, paving the pathway for potential cartilage regeneration. Raabe et al. found that hydrolyzed fish collagen as well as the growth factor TGFB1 induced proteoglycan and collagen fiber synthesis [44]. Fish collagen also induced chondrogenic differentiation [44]. Similarly, Bourdon et al. investigated the effects of three collagen hydrolysates from fish skin and cartilage on the breakdown of chondrocytes [29]. The study found that 0.5, 50 and 100 µg/mL collagen hydrolysates elevated the level of collagen type I and II collagen. In addition, collagen-treated cells had decreased expression of protease markers known to be involved in OA development, Htra1, Mmp103, Adamts5 and Cox2 [29]. Ohnishi et al. also found that rabbits administered a combination of fish collagen peptides and glucosamine were protected from induced cartilage degradation (OA), whereas control groups developed OA [45]. It was found that fish collagen peptide and glucosamine, which are present in large amounts in connective tissue, and help maintain cartilage structure and integrity, had some protective effects alone, but their combined effects provided the most protection against OA [45].
In a histological experiment by Ahmed et al., the effect of collagen from jellyfish sponge scaffolds on the chondrogenicity of bovine cartilage was observed [46]. Chondrogenicity is a complex process involving the proliferation and differentiation of chondroprogenitors and deposition of the extracellular matrix (ECM) [46]. In this experiment, chondrocytes derived from bovine cartilage tissue were seeded on jellyfish scaffolds to evaluate the amount of collagen deposition, and picrosirius red dye was applied to observe the content and orientation of collagen fibers (Figure 2) [46]. They used three different culture media: native tissue (bovine-derived immature cartilage) and bovine cartilage tissue containing chondroprogenitor cells in the presence and absence of transforming growth factor-β1 (TGFβ1) [46]. TGFβ1 has been shown to be an effective growth factor in cartilage formation and is present at high levels in healthy cartilage, but its level is greatly reduced in the cartilage of OA patients [46]. Staining results showed that in native bovine tissue, collagen fibers are mainly located on the tissue surface, but in chondrogenic culture, both at the tissue surface and in deeper areas, deposition of collagen fibers is more visible. Moreover, the addition of TGFβ1 to the culture medium further contributed to increasing the deposition of collagen fibers [46]. Taken together, the present data support the application of marine collagen in cartilage regeneration.
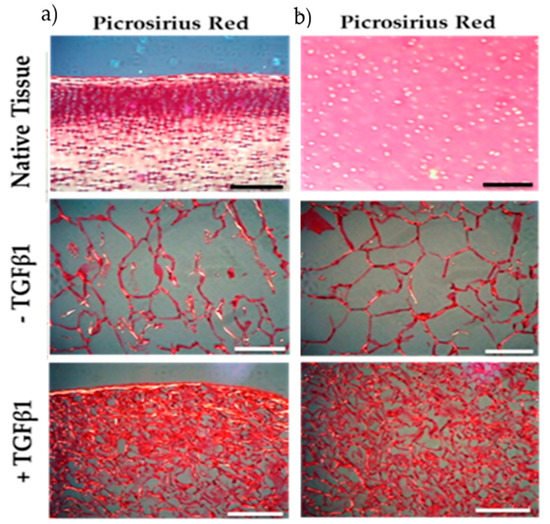
Figure 2. Histologic results show the amount and orientation of collagen fibers in native bovine tissue (containing immature chondrocytes) and chondrogenic tissue derived from bovine cartilage in the presence and absence of TGFβ1. Tissues are seeded on jellyfish collagen scaffolds. Collagen fibers were identified by picrosirius red staining. (a) Surface (b) center of scaffolds. Scale bars: 0.1 mm [46].
3. Advantages and Limitations Associated with Marine Collagen Use
Marine resources of collagen have many advantages over land animals and other sources. Not only are they available in abundance, have no religious constraints and are easily accessible, there have been few reported toxic effects at effective doses [6][7]. This is significant as a major source of collagen is from cattle, which have a risk of transmitting highly dangerous BSE and TSE [3][9]. In addition to its promising safety profile, the use of marine collagen is environmentally friendly. Fish skin, bones, and scales are vast sources of collagen, yet they are often discarded by seafood processing industries [4]. By using marine collagen, useful waste is reduced, and no further organisms are harmed in the isolation of collagen. Furthermore, collagen has a variety of applications in many fields, such as drug delivery, wound healing, skin aging, and tissue regeneration. Marine collagen was also shown to be as effective as sham collagen. In the example mentioned above, marine collagen was as effective as the currently administered antioxidant BHT [47]. In addition, other comparisons of sponge collagen membrane versus polyurethane membrane on healing of grant donor sites depicted that collagen use significantly increased wound healing quality and reduced healing time [48]. Marine collagens are also hydrolyzed more easily than mammalian collagen, which makes them more suited for further processing into peptide derivatives [49]. Furthermore, collagen has both structural and functional properties that make it a natural substrate for cell attachment, growth, and differentiation [50]. It is important to note, however, that although minor, some limitations do exist. It has been shown that marine collagen is less thermally stable than collagen from bovines, as they have fewer proline and hydroxyproline residues [51]. Additionally, most studies have investigated marine collagen’s efficacy in vitro or in animal models; however, more studies are needed that investigate the efficacy and potential adverse effects of marine collagen on human skin. Overall, the few limitations of marine collagen are strongly outweighed by their wide variety of benefits.
References
- Sugiura, H.; Yunoki, S.; Kondo, E.; Ikoma, T.; Tanaka, J.; Yasuda, K. In Vivo Biological Responses and Bioresorption of Tilapia Scale Collagen as a Potential Biomaterial. J. Biomater. Sci. Polym. Ed. 2009, 20, 1353–1368.
- Cheng, X.; Shao, Z.; Li, C.; Yu, L.; Raja, M.A.; Liu, C. Isolation, Characterization and Evaluation of Collagen from Jellyfish Rhopilema Esculentum Kishinouye for Use in Hemostatic Applications. PLoS ONE 2017, 12, e0169731.
- Widdowson, J.P.; Picton, A.J.; Vince, V.; Wright, C.J.; Mearns-Spragg, A. In Vivo Comparison of Jellyfish and Bovine Collagen Sponges as Prototype Medical Devices: In Vivo comparison of jellyfish and bovine collagen sponges. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 1524–1533.
- Coppola, D.; Oliviero, M.; Vitale, G.A.; Lauritano, C.; D’Ambra, I.; Iannace, S.; de Pascale, D. Marine Collagen from Alternative and Sustainable Sources: Extraction, Processing and Applications. Mar. Drugs 2020, 18, 214.
- Silvipriya, K.; Kumar, K.; Bhat, A.; Kumar, B.; John, A.; Lakshmanan, P. Collagen: Animal Sources and Biomedical Application. J. Appl. Pharm. Sci. 2015, 5, 123–127.
- Shavandi, A.; Hou, Y.; Carne, A.; McConnell, M.; Bekhit, A.E.-D.A. Marine Waste Utilization as a Source of Functional and Health Compounds. Adv. Food Nutr. Res. 2019, 87, 187–254.
- Hou, Y.; Shavandi, A.; Carne, A.; Bekhit, A.A.; Ng, T.B.; Cheung, R.C.F.; Bekhit, A.E.A. Marine Shells: Potential Opportunities for Extraction of Functional and Health-Promoting Materials. Crit. Rev. Environ. Sci. Technol. 2016, 46, 1047–1116.
- Karim, A.A.; Bhat, R. Gelatin Alternatives for the Food Industry: Recent Developments, Challenges and Prospects. Trends Food Sci. Technol. 2008, 19, 644–656.
- Karim, A.A.; Bhat, R. Fish Gelatin: Properties, Challenges, and Prospects as an Alternative to Mammalian Gelatins. Food Hydrocoll. 2009, 23, 563–576.
- Yamada, S.; Yamamoto, K.; Ikeda, T.; Yanagiguchi, K.; Hayashi, Y. Potency of Fish Collagen as a Scaffold for Regenerative Medicine. BioMed Res. Int. 2014, 2014, 302932.
- Zhou, T.; Wang, N.; Xue, Y.; Ding, T.; Liu, X.; Mo, X.; Sun, J. Electrospun Tilapia Collagen Nanofibers Accelerating Wound Healing via Inducing Keratinocytes Proliferation and Differentiation. Colloids Surf. B Biointerfaces 2016, 143, 415–422.
- Sampath Kumar, N.S.; Nazeer, R.A.; Jaiganesh, R. Wound Healing Properties of Collagen from the Bone of Two Marine Fishes. Int. J. Pept. Res. Ther. 2012, 18, 185–192.
- Shalaby, M.; Agwa, M.; Saeed, H.; Khedr, S.M.; Morsy, O.; El-Demellawy, M.A. Fish Scale Collagen Preparation, Characterization and Its Application in Wound Healing. J. Polym. Environ. 2020, 28, 166–178.
- Feng, X.; Zhang, X.; Li, S.; Zheng, Y.; Shi, X.; Li, F.; Guo, S.; Yang, J. Preparation of Aminated Fish Scale Collagen and Oxidized Sodium Alginate Hybrid Hydrogel for Enhanced Full-Thickness Wound Healing. Int. J. Biol. Macromol. 2020, 164, 626–637.
- Pal, P.; Srivas, P.K.; Dadhich, P.; Das, B.; Maity, P.P.; Moulik, D.; Dhara, S. Accelerating Full Thickness Wound Healing Using Collagen Sponge of Mrigal Fish (Cirrhinus Cirrhosus) Scale Origin. Int. J. Biol. Macromol. 2016, 93, 1507–1518.
- Wang, J.; Xu, M.; Liang, R.; Zhao, M.; Zhang, Z.; Li, Y. Oral Administration of Marine Collagen Peptides Prepared from Chum Salmon (Oncorhynchus Keta) Improves Wound Healing Following Cesarean Section in Rats. Food Nutr. Res. 2015, 59, 26411.
- Zhang, Z.; Wang, J.; Ding, Y.; Dai, X.; Li, Y. Oral Administration of Marine Collagen Peptides from Chum Salmon Skin Enhances Cutaneous Wound Healing and Angiogenesis in Rats. J. Sci. Food Agric. 2011, 91, 2173–2179.
- Chen, J.; Gao, K.; Liu, S.; Wang, S.; Elango, J.; Bao, B.; Dong, J.; Liu, N.; Wu, W. Fish Collagen Surgical Compress Repairing Characteristics on Wound Healing Process In Vivo. Mar. Drugs 2019, 17, 33.
- Ito, N.; Seki, S.; Ueda, F. Effects of Composite Supplement Containing Collagen Peptide and Ornithine on Skin Conditions and Plasma IGF-1 Levels—A Randomized, Double-Blind, Placebo-Controlled Trial. Mar. Drugs 2018, 16, 482.
- De Luca, C.; Mikhal’chik, E.V.; Suprun, M.V.; Papacharalambous, M.; Truhanov, A.I.; Korkina, L.G. Skin Antiageing and Systemic Redox Effects of Supplementation with Marine Collagen Peptides and Plant-Derived Antioxidants: A Single-Blind Case-Control Clinical Study. Oxid. Med. Cell. Longev. 2016, 2016, 4389410.
- Allouche, M.; Hamdi, I.; Nasri, A.; Harrath, A.H.; Mansour, L.; Beyrem, H.; Boufahja, F. Laboratory Bioassay Exploring the Effects of Anti-Aging Skincare Products on Free-Living Marine Nematodes: A Case Study of Collagen. Environ. Sci. Pollut. Res. 2020, 27, 11403–11412.
- Kim, C.-R.; Kim, Y.-M.; Lee, M.-K.; Kim, I.-H.; Choi, Y.-H.; Nam, T.-J. Pyropia Yezoensis Peptide Promotes Collagen Synthesis by Activating the TGF-β/Smad Signaling Pathway in the Human Dermal Fibroblast Cell Line Hs27. Int. J. Mol. Med. 2017, 39, 31–38.
- Nomura, Y.; Oohashi, K.; Watanabe, M.; Kasugai, S. Increase in Bone Mineral Density through Oral Administration of Shark Gelatin to Ovariectomized Rats. Nutrition 2005, 21, 1120–1126.
- Liu, C.; Sun, J. Potential Application of Hydrolyzed Fish Collagen for Inducing the Multidirectional Differentiation of Rat Bone Marrow Mesenchymal Stem Cells. Biomacromolecules 2014, 15, 436–443.
- Yamada, S.; Nagaoka, H.; Terajima, M.; Tsuda, N.; Hayashi, Y.; Yamauchi, M. Effects of Fish Collagen Peptides on Collagen Post-Translational Modifications and Mineralization in an Osteoblastic Cell Culture System. Dent. Mater. J. 2013, 32, 88–95.
- Elango, J.; Robinson, J.; Zhang, J.; Bao, B.; Ma, N.; de Val, J.E.M.S.; Wu, W. Collagen Peptide Upregulates Osteoblastogenesis from Bone Marrow Mesenchymal Stem Cells through MAPK- Runx2. Cells 2019, 8, 446.
- Capati, M.L.F.; Nakazono, A.; Yamamoto, K.; Sugimoto, K.; Yanagiguchi, K.; Yamada, S.; Hayashi, Y. Fish Collagen Promotes the Expression of Genes Related to Osteoblastic Activity. Int. J. Polym. Sci. 2016, 2016, 5785819.
- Hsu, H.-H.; Uemura, T.; Yamaguchi, I.; Ikoma, T.; Tanaka, J. Chondrogenic Differentiation of Human Mesenchymal Stem Cells on Fish Scale Collagen. J. Biosci. Bioeng. 2016, 122, 219–225.
- Bourdon, B.; Contentin, R.; Cassé, F.; Maspimby, C.; Oddoux, S.; Noël, A.; Legendre, F.; Gruchy, N.; Galéra, P. Marine Collagen Hydrolysates Downregulate the Synthesis of Pro-Catabolic and Pro-Inflammatory Markers of Osteoarthritis and Favor Collagen Production and Metabolic Activity in Equine Articular Chondrocyte Organoids. Int. J. Mol. Sci. 2021, 22, 580.
- Hadjiargyrou, M.; Lombardo, F.; Zhao, S.; Ahrens, W.; Joo, J.; Ahn, H.; Jurman, M.; White, D.W.; Rubin, C.T. Transcriptional Profiling of Bone Regeneration. J. Biol. Chem. 2002, 277, 30177–30182.
- Armiento, A.R.; Hatt, L.P.; Sanchez Rosenberg, G.; Thompson, K.; Stoddart, M.J. Functional Biomaterials for Bone Regeneration: A Lesson in Complex Biology. Adv. Funct. Mater. 2020, 30, 1909874.
- Dimitriou, R.; Jones, E.; McGonagle, D.; Giannoudis, P.V. Bone Regeneration: Current Concepts and Future Directions. BMC Med. 2011, 9, 66.
- Cicciù, M. Real Opportunity for the Present and a Forward Step for the Future of Bone Tissue Engineering. J. Craniofac. Surg. 2017, 28, 592–593.
- Xu, Y.; Han, X.; Li, Y. Effect of Marine Collagen Peptides on Long Bone Development in Growing Rats. J. Sci. Food Agric. 2010, 90, 1485–1491.
- Meng, K.; Chen, L.; Xia, G.; Shen, X. Effects of Zinc Sulfate and Zinc Lactate on the Properties of Tilapia (Oreochromis Niloticus) Skin Collagen Peptide Chelate Zinc. Food Chem. 2021, 347, 129043.
- Yamada, S.; Yoshizawa, Y.; Kawakubo, A.; Ikeda, T.; Yanagiguchi, K.; Hayashi, Y. Early Gene and Protein Expression Associated with Osteoblast Differentiation in Response to Fish Collagen Peptides Powder. Dent. Mater. J. 2013, 32, 233–240.
- Diogo, G.; López-Senra, E.; Pirraco, R.; Canadas, R.; Fernandes, E.; Serra, J.; Pérez-Martín, R.; Sotelo, C.; Marques, A.; González, P.; et al. Marine Collagen/Apatite Composite Scaffolds Envisaging Hard Tissue Applications. Mar. Drugs 2018, 16, 269.
- Flaig, I.; Radenković, M.; Najman, S.; Pröhl, A.; Jung, O.; Barbeck, M. In Vivo Analysis of the Biocompatibility and Immune Response of Jellyfish Collagen Scaffolds and Its Suitability for Bone Regeneration. Int. J. Mol. Sci. 2020, 21, 4518.
- Rachmawati, R.; Hidayat, M.; Permatasari, N.; Widyarti, S. Potential Effect of Jellyfish Aurelia Aurita Collagen Scaffold Induced Alveolar Bone Regeneration in Periodontal Disease. Syst. Rev. Pharm. 2021, 12, 1397–1404.
- Lin, Z.; Solomon, K.L.; Zhang, X.; Pavlos, N.J.; Abel, T.; Willers, C.; Dai, K.; Xu, J.; Zheng, Q.; Zheng, M. In Vitro Evaluation of Natural Marine Sponge Collagen as a Scaffold for Bone Tissue Engineering. Int. J. Biol. Sci. 2011, 7, 968–977.
- Green, D.; Howard, D.; Yang, X.; Kelly, M.; Oreffo, R.O.C. Natural Marine Sponge Fiber Skeleton: A Biomimetic Scaffold for Human Osteoprogenitor Cell Attachment, Growth, and Differentiation. Tissue Eng. 2003, 9, 1159–1166.
- Glyn-Jones, S.; Palmer, A.J.R.; Agricola, R.; Price, A.J.; Vincent, T.L.; Weinans, H.; Carr, A.J. Osteoarthritis. Lancet 2015, 386, 376–387.
- Goldring, M.B.; Otero, M. Inflammation in Osteoarthritis. Curr. Opin. Rheumatol. 2011, 23, 471–478.
- Raabe, O.; Reich, C.; Wenisch, S.; Hild, A.; Burg-Roderfeld, M.; Siebert, H.-C.; Arnhold, S. Hydrolyzed Fish Collagen Induced Chondrogenic Differentiation of Equine Adipose Tissue-Derived Stromal Cells. Histochem. Cell Biol. 2010, 134, 545–554.
- Ohnishi, A.; Osaki, T.; Matahira, Y.; Tsuka, T.; Imagawa, T.; Okamoto, Y.; Minami, S. Evaluation of the Chondroprotective Effects of Glucosamine and Fish Collagen Peptide on a Rabbit ACLT Model Using Serum Biomarkers. J. Vet. Med. Sci. 2013, 75, 421–429.
- Ahmed, Z.; Powell, L.C.; Matin, N.; Mearns-Spragg, A.; Thornton, C.A.; Khan, I.M.; Francis, L.W. Jellyfish Collagen: A Biocompatible Collagen Source for 3D Scaffold Fabrication and Enhanced Chondrogenicity. Mar. Drugs 2021, 19, 405.
- Chi, C.-F.; Hu, F.-Y.; Wang, B.; Ren, X.-J.; Deng, S.-G.; Wu, C.-W. Purification and Characterization of Three Antioxidant Peptides from Protein Hydrolyzate of Croceine Croaker (Pseudosciaena Crocea) Muscle. Food Chem. 2015, 168, 662–667.
- Horch, R.E.; Stark, G.B. Comparison of The Effect of A Collagen Dressing and A Polyurethane Dressing on The Healing of Split Thickness Skin Graft (Stsg) Donor Sites. Scand. J. Plast. Reconstr. Surg. Hand Surg. 1998, 32, 407–414.
- Caruso, G.; Floris, R.; Serangeli, C.; Di Paola, L. Fishery Wastes as a Yet Undiscovered Treasure from the Sea: Biomolecules Sources, Extraction Methods and Valorization. Mar. Drugs 2020, 18, 622.
- Fratzl, P.; Misof, K.; Zizak, I.; Rapp, G.; Amenitsch, H.; Bernstorff, S. Fibrillar Structure and Mechanical Properties of Collagen. J. Struct. Biol. 1998, 122, 119–122.
- Gauza-Włodarczyk, M.; Kubisz, L.; Mielcarek, S.; Włodarczyk, D. Comparison of Thermal Properties of Fish Collagen and Bovine Collagen in the Temperature Range 298–670 K. Mater. Sci. Eng. C 2017, 80, 468–471.
More
