Despite growing evidence that demonstrate adverse effects of artificial light at night (ALAN) on many species, relatively little is known regarding its effects on brain plasticity in birds. We recently showed that although ALAN increases cell proliferation in brains of birds, neuronal densities in two brain regions decreased, indicating neuronal death, which might be due to mortality of newly produced neurons or of existing ones. Therefore, in the present study we studied the effect of long-term ALAN on the recruitment of newborn neurons into their target regions in the brain. Accordingly, we exposed zebra finches (Taeniopygia guttata) to 5 lux ALAN, and analysed new neuronal recruitment and total neuronal densities in several brain regions. We found that ALAN increased neuronal recruitment, possibly as a compensatory response to ALAN-induced neuronal death, and/or due to increased nocturnal locomotor activity caused by sleep disruption. Moreover, ALAN also had a differential temporal effect on neuronal densities, because hippocampus was more sensitive to ALAN and its neuronal densities were more affected than in other brain regions. Nocturnal melatonin levels under ALAN were significantly lower compared to controls, indicating that very low ALAN intensities suppress melatonin not only in nocturnal, but also in diurnal species.
- artificial light at night (ALAN)
- brain plasticity
- new neuronal recruitment
- neuronal densities
- hippocampus (HC)
- Medial striatum (MSt)
- Nidopallium caudale (NC)
- melatonin
- birds
- zebra finches (Taeniopygia guttata).
1. Introduction
Despite a growing number of studies that demonstrate adverse effects of artificial light at night (ALAN) on the behaviour and physiology of many species (e.g., reviewed in [1][2]), still, in birds relatively little is known regarding its effects on brain plasticity. The few existing studies report a decrease in the numbers of newly formed neurons in the hippocampus (HC) of Indian house crows (
) [3], and a decrease in soma size of neurons in the HC and the lateral caudal nidopallium (NCL) in this species, suggesting reduced neuronal plasticity [4]. We have also studied the effect of ALAN on neuronal plasticity in another diurnal species, zebra finches (
), which have excellent visual abilities [5] and their physiology, reproduction and survival are greatly affected by circadian and circannual rhythms [6][7]. Our recent study [8] was the first to demonstrate that ALAN increases cell proliferation in brains of diurnal birds. More specifically, we found, in female zebra finches, that ecologically relevant intensities (0.5, 1.5, and 5 lux) of ALAN significantly increased cell proliferation in the ventricular zone (VZ), from which, in birds, the new cells migrate to the telencephalon, differentiate into neurons and settle in various brain regions [9][10], where they are recruited into functional circuits [11].
However, we also recorded a decrease in total neuronal densities under ALAN exposure in the nidopallium caudale (NC), the medial striatum (MSt), and the HC, compared with controls [8]. The NC contains auditory relays [12], is involved in vocal communication, and in the integration of auditory information [13][14]; the MSt is part of the avian somatomotor basal ganglia [15] and is linked to visual perception and associative learning [16][17][18], and the HC processes spatial information [19][20] and plays a role in stress response [21]. In two of these regions, the NC and the MSt, the decrease in total neuronal densities was significant. This decrease in total neuronal densities, despite the increase in proliferation, suggests a net neuronal death. Taken together, the findings in our previous study [8] add to the notion of the deleterious effects of ALAN.
Two equally possible hypotheses might explain the results of our previous study, which indicated neuronal death despite the increase of cell proliferation in the VZ: (1) many of the new neurons produced in the VZ died under ALAN conditions, while migrating to their target destinations in the brain, hence only the relatively few that survived actually reached these target regions and had been recruited there. (2) ALAN causes a significant reduction in existing neurons, so that even increased proliferation and probable consequent increased influx of new neurons did not compensate for the loss due to neuronal death. Accordingly, our current work aims to help differentiate between these hypotheses, by investigating whether ALAN affects the recruitment of the new born neurons into their target regions in the brain. We exposed female zebra finches to ALAN for 7 weeks, and compared the recruitment of new neurons and total neuronal densities in selected brain regions (MSt, NC, HC) with control birds that were kept under dark nights. The ALAN intensity that we used was 5 lux, as it is ecologically relevant to birds [22][23] and had the greatest effect on cell proliferation in the VZ in our previous study [8]. In addition, in the present study, ALAN exposure was longer than in our previous one, to allow enough time for the new neurons to arrive at their final destination, and to check whether the previous exposure to ALAN was too short for the brain to reach an equilibrium between neuronal death and compensatory recruitment of new neurons. We also measured plasma melatonin (MEL) concentrations as a key output of the central clock and which we previously showed was suppressed in response to a 3-week ALAN exposure in female zebra finches [8].
2. ALAN Increases New Neuronal Recruitment in the MSt, HC, and NC
In various brain regions in birds, older neurons are constantly being replaced by new ones [10][24], and the assumption is that the total number of neurons within a brain region remains constant. However, in our previous study [8] we found, that despite an increase in cell proliferation in the VZ under ALAN conditions, total neuronal densities in target regions decreased. We suggested that this might be because ALAN impairs the brain’s ability to keep the total number of neurons within a certain region constant, due to death of the older, already-existing neurons in that region, or death of the new neurons, during their migration from the VZ and their recruitment into that region, or both. The present results, which show that new neuronal recruitment into the MSt, HC and NC was significantly increased (
), indicate that ALAN does not impair neuronal recruitment.
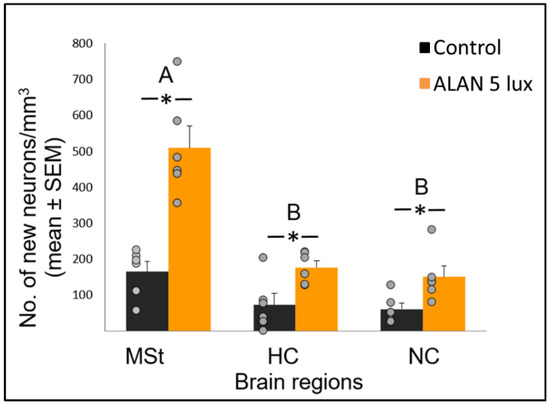
Figure 1. Neuronal recruitment (number of new neurons per mm3; mean ± SE) in three brain regions—medial striatum (MSt), hippocampus (HC) and nidopallium caudale (NC), of birds exposed to 5 lux ALAN and controls that remained in dark nights. Grey dots indicate individual data points, * indicates a significant difference between groups (p < 0.05), and different letters indicate significant differences between brain regions (N = 6 birds/group).
Neuronal recruitment was greater in the MSt compared to NC and HC in both control and ALAN groups (
). In addition, the increase under ALAN relative to control in the MSt was 3-fold, compared with 2.5-fold in the other two regions, with marginally significant (
= 0.052) group x region interaction, indicating a possible differential effect of ALAN on neuronal recruitment into different brain regions. The MSt is part of the somatomotor basal ganglia in birds, and also plays a role in avoidance behaviour [25], associative learning [26] and in visual perception [16][17][18]. Analysis of medial and lateral sub-regions of the MSt separately, revealed that ALAN increased neuronal recruitment in both (
). The mMSt receives dopaminergic inputs from the ventral tegmental region that is responsible for homeostatic and reflexive pathways [15][27][28], whereas the lMSt receives dopaminergic inputs from the substantia nigra pars compacta [29][30] and pallial input from regions involved in somatosensory, visual, auditory, and motor function [15][31][32]. Thus, our results indicate that ALAN affects both viscerolimbic and somatosensory functions. Under control conditions lMSt recruits fewer new neurons in comparison to mMSt (
). To the best of our knowledge this is novel information that has not been reported earlier. Similarly, in an earlier study, we observed that the survival of new neurons in the NC is influenced by their position within this region [33][34]. This finding led us then to suggest that when sampling a large region, questions about neuronal recruitment have to take into account also the spatial distribution of the new neurons within that region [35]. Our current findings in MSt support this suggestion. In addition, in our current study, exposure to ALAN significantly increased neuronal recruitment in both sub-regions of MSt, but it was more evident in lMSt than in mMSt. This differential increase suggests that mMSt is more resilient to ALAN than lMSt, which might be related to the functions of these sub-regions: if ALAN causes the birds to be more awake and active during the nights, this might affect lMSt, because it is connected to the substantia nigra that plays an important role in movement.
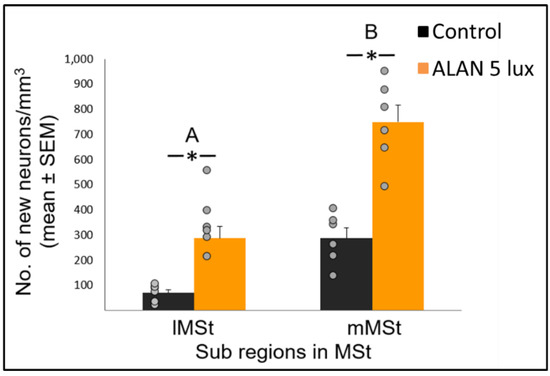
Figure 2. New neuronal recruitment (number of new neurons per mm3; mean ± SE) in the lateral (lMSt) and medial (mMSt) sub-regions of the medial striatum (MSt). Control birds were exposed to dark night whereas the experimental group was exposed to 5 lux ALAN. Grey dots indicate the individual data points, * indicates a significant difference between groups (p < 0.05), and different letters indicate significant difference between MSt sub-regions (N = 6 birds/group).
In the HC, which is involved in the processing of spatial information [19][20], the significant increase in the number of new neurons that were recruited under ALAN is contrary to the decrease that was found in Indian house crows [3]. However, these crows were acclimated, after being caught from the wild, only for a single week, shorter than the recommended three weeks to ensure thermogenic [36] and physiological [37] acclimation in birds. In addition, the crows were exposed to ALAN only for 10 days, and the marker that was used to detect new neurons was doublecortin, which is expressed in postmitotic migrating and still differentiating newborn neurons [38]. Therefore, it could be that the finding in crows represents a transient stage of young, not matured neurons, which have not been incorporated yet in their final destinations. The increase in the number of new neurons that we had found is also opposite to the decrease that was found in nocturnal mammals under ALAN conditions [39][40] and supports our suggestion that ALAN affects nocturnal and diurnal species differently [8]. It is unlikely that this increase was mediated by a greater demand for spatial memory, since both groups were acclimated to their cages for three weeks prior to the onset of the experiment, and were kept there throughout ALAN exposure, until they were killed. HC is also related to stress, however stress is known to decrease rather than increase neuronal recruitment, both in mammals [41] and birds [42]. Therefore, based on the current existing evidence in the literature, it is unlikely that stress can explain our observation of increased neuronal recruitment under ALAN. We therefore suggest that the increase in the HC might be a pathological response to ALAN, which changes the normal ratio between the influx of new neurons and death of others in this brain region. To test this possibility, in a currently ongoing study we also record apoptosis under ALAN conditions.
In the NC we also observed an increase in neuronal recruitment under ALAN conditions (
). NC is the centre for auditory relays and vocal communication [12], and because MEL receptors occur in high density in major brain components of the avian auditory and visual systems [43], it could be that there is a relation between the increased neuronal recruitment and the decrease in nocturnal MEL under ALAN (
3). The low nocturnal MEL levels can facilitate higher activity during the night, a possibility that is supported by evidence of a negative correlation between MEL levels and locomotor activity in birds [44]. Moreover, locomotion activity is known to increase neuronal plasticity [45] and might also influence processing of auditory information. The possibility that our birds were more active during the nights under ALAN conditions might also result from sleep disruption, which occurs in birds that are exposed to light pollution [46]. We could not validate this hypothesis in our current experimental setup, but in a follow-up experiment we will also record nocturnal locomotion and sleep behaviour of control vs. ALAN-exposed birds.
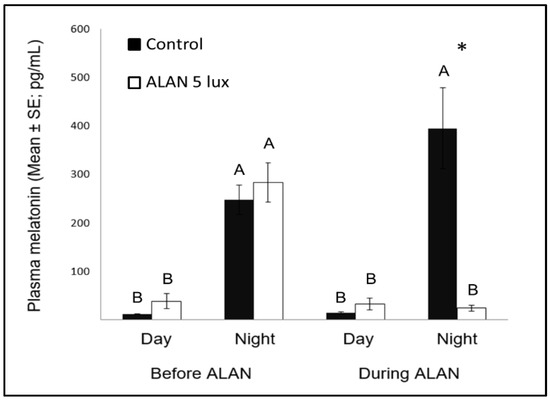
Figure 3. Day and night plasma melatonin levels (pg/mL; Mean ± SE) before and after exposure to 5 lux ALAN, compared with controls that remained under dark nights. * indicates significant difference between groups (p < 0.05) and different letters indicate significant differences between day and night (N = 6 birds/group).
3. A Possible Temporal Differential Effect of ALAN on Total Neuronal Densities in Various Brain Regions
In view that ALAN increased neuronal recruitment, as well as cell proliferation in the VZ, it remains to explain the reduction of total neuronal densities observed in our previous study [8]. It is possible that ALAN causes a significant reduction in existing neurons, so that even the increased proliferation and the probable consequent increased influx of new neurons could not compensate for the loss, due to greater neuronal death. We were aware that the 3-week exposure in our previous study might be too short to enable such compensation and retain constant neuronal densities. Therefore, in our present study we exposed birds to ALAN for seven weeks and found that MSt and NC maintained their total neuronal densities compared to controls. This suggests that the effect of ALAN is time-dependent, so that under longer ALAN exposure there is enough time for the brain to balance between the increase in new neurons (
and
) and the death of older ones.
It is interesting to note that the temporal effect of ALAN on neuronal densities seems to be region-specific. This is because HC responded to ALAN differently than MSt and NC, both in the short-term [8], as well as in the long-term ALAN exposures (our present study). In the short-term (3 weeks), neuronal densities decreased in both the MSt and the NC, whereas no significant change was observed in the HC, similar to what had been found when Indian house crows were exposed to an even shorter ALAN duration (two weeks) [4]. However, in the long-term exposure of our current study (seven weeks), total neuronal densities in the HC increased compared to controls, whereas no changes were observed in MSt and NC. Thus, we suggest that while neuronal densities in the MSt and the NC are negatively affected during short-term ALAN exposure, these regions manage to compensate under long-term ALAN exposure. However, the HC exhibits an opposite pattern: during a short-term ALAN exposure neuronal densities are retained, but under long-term ALAN exposure they are significantly higher compared with control. If we assume that long-term ALAN exposure is the ecologically relevant duration, then it can be suggested that the differential temporal effect of ALAN on total neuronal densities renders some brain regions (as the MSt and NC) more resilient to it than others (as HC), because the former ones manage to retain their normal densities, whereas the density of the latter one departs from the normal level.
4. Possible Relation between Melatonin and Neuronal Recruitment in Birds
Nocturnal MEL levels under ALAN conditions were significantly lower compared to control birds that were exposed to dark nights (
3), similar to previous reports in zebra finches [8][47], as well as in other bird species [23], diurnal mammals [48], and fish [49]. Evidently, very low ALAN intensities can suppress MEL biosynthesis not only in nocturnal [50], but also in diurnal species.
Another interesting comparison is the possible effect of MEL on neuronal survival in birds and other species. Our previous study [8] found that ALAN caused lower levels of nocturnal MEL, as well as decreased neuronal densities in some brain regions (MSt and NC), suggesting a possible relation between the two variables, which is in line with findings in mice, of a positive correlation between MEL and neuronal survival [51]. However, our present study does not support such correlation, because after a longer ALAN exposure neuronal densities in these regions were no longer different from those found in controls, although nocturnal MEL levels remained low. These different patterns between mice (which are nocturnal animals) and our birds (which are diurnal ones), supports our suggestion [8], that the physiological interpretation of ALAN might be different in nocturnal and diurnal animals.
5. Conclusions and Future Directions
Our study provides new evidence that ALAN increases new neuronal recruitment in the avian brain. We suggest that this increase might be a result of a compensatory response to neuronal death caused by ALAN, and/or due to increased locomotor activity during the night caused by sleep disruption. Our findings also indicate that ALAN has a differential temporal effect on various brain regions, some of them are more resilient and retain their normal numbers of neurons under long-term ALAN exposure, while others are more sensitive, and their neuronal densities are more affected. Further research is required to fully understand the effects of ALAN on neuronal plasticity in the avian brain, and therefore we intend to also record the effect of ALAN on apoptosis, and monitor the birds’ nocturnal locomotor activity and sleep. Finally, we plan to further investigate the intriguing indication for a possible temporal effect of ALAN on neuronal mechanisms in the brain, by studying these features in birds after a life-long ALAN exposure.
References
- Grubisic, M.; Haim, A.; Bhusal, P.; Dominoni, D.M.; Gabriel, K.; Jechow, A.; Kupprat, F.; Lerner, A.; Marchant, P.; Riley, W.; et al. Light pollution, circadian photoreception, and melatonin in vertebrates. Sustainability 2019, 11, 6400.
- Hölker, F.; Wolter, C.; Perkin, E.K.; Tockner, K. Light pollution as a biodiversity threat. Trends Ecol. Evol. 2010, 25, 681–682.
- Taufique, S.T.; Prabhat, A.; Kumar, V. Illuminated night alters hippocampal gene expressions and induces depressive-like responses in diurnal corvids. Eur. J. Neurosci. 2018, 48, 3005–3018.
- Taufique, S.T.; Prabhat, A.; Kumar, V. Light at night affects hippocampal and nidopallial cytoarchitecture: Implication for impairment of brain function in diurnal corvids. J. Exp. Zool. Part A 2019, 331, 149–156.
- Goldsmith, T.H.; Goldsmith, T.H. Optimization, constraint and history in the evolution of eyes. Q. Rev. Biol. 1990, 65, 281–322.
- Gaston, K.J.; Visser, M.E.; Hölker, F. The biological impacts of artificial light at night: The research challenge. Philos. Trans. R. Soc. B 2015, 370, 20140133.
- Dawson, A.; King, V.M.; Bentley, G.E.; Ball, G.F. Photoperiodic control of seasonality in birds. J. Biol. Rhythm. 2001, 16, 365–380.
- Moaraf, S.; Vistoropsky, Y.; Pozner, T.; Heiblum, R.; Okuliarová, M.; Zeman, M.; Barnea, A. Artificial light at night affects brain plasticity and melatonin in birds. Neurosci. Lett. 2020, 716, 134639.
- Kirn, J.R.; Schwabl, H. Photoperiod regulation of neuron death in the adult canary. J. Neurobiol. 1997, 33, 223–231.
- Scharff, C.; Kirn, J.R.; Grossman, M.; Macklis, J.D.; Nottebohm, F. Targeted neuronal death affects neuronal replacement and vocal behavior in adult songbirds. Neuron 2000, 25, 481–492.
- Paton, J.A.; Nottebohm, F. Neurons generated in the adult brain are recruited into functional circuits. Science 1984, 225, 1046–1048.
- Vates, G.E.; Broome, B.M.; Mello, C.V.; Nottebohm, F. Auditory pathways of caudal telencephalon and their relation to the song system of adult male zebra finches (Taenopygia guttata). J. Comp. Neurol. 1996, 366, 613–642.
- Mello, C.V.; Clayton, D.F. Song-induced ZENK gene expression in auditory pathways of songbird brain and its relation to the song control system. J. Neurosc. 1994, 14, 6652–6666.
- Mello, C.V.; Vates, E.; Okuhata, S.; Nottebohm, F. Descending auditory pathways in the adult male zebra finch (Taeniopygia guttata). J. Comp. Neurol. 1998, 395, 137–160.
- Kuenzel, W.J.; Medina, L.; Csillag, A.; Perkel, D.J.; Reiner, A. The avian subpallium: New insights into structural and functional subdivisions occupying the lateral subpallial wall and their embryological origins. Brain Res. 2011, 1424, 67–101.
- Watanabe, S. Effects of lobus parolfactorius lesions on repeated acquisition of spatial discrimination in pigeons. Brain Behav. Evol. 2001, 58, 333–342.
- Matsushima, T.; Izawa, E.I.; Aoki, N.; Yanagihara, S. The mind through chick eyes: Memory, cognition and anticipation. Zool. Sci. 2003, 20, 395–408.
- Reiner, A.; Perkel, D.J.; Bruce, L.L.; Butler, A.; Csillag, A.; Kuenzel, W.; Medina, L.; Paxinos, G.; Shimizu, T.; Striedter, G.; et al. The avian brain nomenclature forum: Terminology for a new century in comparative neuroanatomy. J. Comp. Neurol. 2004, 473, E1.
- Sherry, D.F.; Vaccarino, A.L.; Buckenham, K.; Herz, R.S. The hippocampal complex of food-storing birds. Brain Behav. Evol. 1989, 34, 308–317.
- Shettleworth, S.J. Spatial memory in food-storing birds. Philos. Trans. R. Soc. B 1990, 329, 143–151.
- Smulders, T.V. The avian hippocampal formation and the stress response. Brain Behav. Evol. 2017, 90, 81–91.
- Da Silva, A.; Samplonius, J.M.; Schlicht, E.; Valcu, M.; Kempenaers, B. Artificial night lighting rather than traffic noise affects the daily timing of dawn and dusk singing in common European songbirds. Behav. Ecol. 2014, 25, 1037–1047.
- De Jong, M.; Jening, L.; Ouyang, J.Q.; van Oers, K.; Spoelstra, K.; Visser, M.E. Dose-dependent responses of avian daily rhythms to artificial light at night. Physiol. Behav. 2016, 155, 172–179.
- Kirn, J.; Loughlin, B.O.; Kasparian, S.; Nottebohm, F. Cell death and neuronal recruitment in the high vocal center of adult male canaries are temporally related to changes in song. Proc. Natl. Acad. Sci. USA 1994, 91, 7844–7848.
- Jarvis, E.D.; Güntürkün, O.; Bruce, L.; Csillag, A.; Karten, H.; Kuenzel, W.; Medina, L.; Paxinos, G.; Perkel, D.J.; Shimizu, T.; et al. Avian brains and a new understanding of vertebrate brain evolution. Nat. Rev. Neurosci. 2005, 6, 151–159.
- Marzluff, J.M.; Bowman, R.; Donnelly, R. (Eds.) Avian Ecology and Conservation in an Urbanizing World; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Medina, L.; Reiner, A. Distribution of choline acetyltransferase immunoreactivity in the pigeon brain. J. Comp. Neurol. 1994, 342, 497–537.
- Reiner, A.; Karle, E.J.; Anderson, K.D.; Medina, L. Catecholaminergic perikarya and fivers in the avian nervous system. In Phylogeny and Development of Catecholaminergic Systems in the CNS of Vertebrates; Smeets, W.J., Reiner, A., Eds.; Cambridge University: Cambridge, UK, 1994; pp. 135–181. [Google Scholar]
- Metzger, M.; Toledo, C.; Braun, K. Serotonergic innervation of the telencephalon in the domestic chick. Brain Res. Bull. 1996, 52, 163–174.
- Reiner, M.A.; Medina, L.; Veenman, C.L. Structural and functional evolution of the basal ganglia in vertebrates. Brain Res. Rev. 1998, 28, 235–285.
- Veenman, C.L.; Wild, J.M.; Reiner, A. Organization of the avian “corticostriatal” projection system: A retrograde and anterograde pathway tracing study in pigeons. J. Comp. Neurol. 1995, 354, 87–126.
- Wild, J.M. Descending projections of the songbird nucleus robustus archistriatalis. J. Comp. Neurol. 1993, 338, 225–241.
- Barnea, A.; Mishal, A.; Nottebohm, F. Social and spatial changes induce multiple survival regimes for new neurons in two regions of the adult brain: An anatomical representation of time? Behav. Brain Res. 2006, 167, 63–74.
- Adar, E.; Nottebohm, F.; Barnea, A. The relationship between nature of social change, age, and position of new neurons and their survival in adult zebra finch brain. J. Neurosci. Res. 2008, 28, 5394–5400.
- Barnea, A.; Pravosudov, V. Birds as a model to study adult neurogenesis: Bridging evolutionary, comparative and neuroethological approaches. Eur. J. Neurosci. 2011, 34, 884–907.
- Heldmaier, G.; Werner, D. Environmental signal processing and adaptation. In Environmental Signal Processing and Adaptation; Springer: Berlin/Heidelberg, Germany, 2003; pp. 1–8. [Google Scholar]
- Maldonado, K.E.; Cavieres, G.; Veloso, C.; Canals, M.; Sabat, P. Physiological responses in rufous-collared sparrows to thermal acclimation and seasonal acclimatization. J. Comp. Physiol. B 2009, 179, 335–343.
- Balthazart, J.; Boseret, G.; Konkle, A.T.; Hurley, L.L.; Ball, G.F. Doublecortin as a marker of adult neuroplasticity in the canary song control nucleus HVC. Eur. J. Neurosci. 2008, 27, 801–817.
- Fujioka, A.; Fujioka, T.; Tsuruta, R.; Izumi, T.; Kasaoka, S.; Maekawa, T. Effects of a constant light environment on hippocampal neurogenesis and memory in mice. Neurosci. Lett. 2011, 488, 41–44.
- Li, D.; Ma, S.; Guo, D.; Cheng, T.; Li, H.; Tian, Y.; Li, J.; Guan, F.; Yang, B.; Wang, J. Environmental circadian disruption worsens neurologic impairment and inhibits hippocampal neurogenesis in adult rats after traumatic brain injury. Cell. Mol. Neurobiol. 2016, 36, 1045–1055.
- Snyder, J.S.; Soumier, A.; Brewer, M.; Pickel, J.; Cameron, H.A. Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature 2011, 476, 458–461.
- Gualtieri, F.; Armstrong, E.A.; Longmoor, G.K.; D’Eath, R.B.; Sandilands, V.; Boswell, T.; Smulders, T.V. Unpredictable chronic mild stress suppresses the incorporation of new neurons at the caudal pole of the chicken Hippocampal Formation. Sci. Rep. 2019, 9, 1–13.
- Brooks, D.S.; Cassone, V.M. Daily and circadian regulation of 2-[125I] iodomelatonin binding in the chick brain. Endocrinology 1992, 131, 1297–1304.
- Hendel, R.C.; Turek, F.W. Suppression of locomotor activity in sparrows by treatment with melatonin. Physiol. Behav. 1987, 21, 275–278.
- Van Praag, H.; Kempermann, G.; Gage, F.H. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat. Neurosci. 1999, 2, 266–270.
- Raap, T.; Pinxten, R.; Eens, M. Light pollution disrupts sleep in free-living animals. Sci. Rep. 2015, 5, 13557.
- Mishra, I.; Knerr, R.M.; Stewart, A.A.; Payette, W.I.; Richter, M.M.; Ashley, N.T. Light at night disrupts diel patterns of cytokine gene expression and endocrine profiles in zebra finch (Taeniopygia guttata). Sci. Rep. 2019, 9, 1–12.
- Robert, K.A.; Lesku, J.A.; Partecke, J.; Chambers, B. Artificial light at night desynchronizes strictly seasonal reproduction in a wild mammal. Proc. R. Soc. B 2015, 282, 20151745.
- Brüning, A.; Hölker, F.; Franke, S.; Preuer, T.; Kloas, W. Spotlight on fish: Light pollution affects circadian rhythms of European perch but does not cause stress. Sci. Total Environ. 2015, 511, 516–522.
- Wren, M.A.; Dauchy, R.T.; Hanifin, J.P.; Jablonski, M.R.; Warfield, B.; Brainard, G.C.; Blask, D.E.; Hill, S.M.; Ooms, T.G.; Bohm, R.P., Jr. Effect of different spectral transmittances through tinted animal cages on circadian metabolism and physiology in Sprague-Dawley rats. J. Am. Assoc. Lab. Anim. Sci. 2014, 53, 44–51. [Google Scholar]
- Kilic, E.; Kilic, Ü.; Bacigaluppi, M.; Guo, Z.; Abdallah, N.B.; Wolfer, D.P.; Reiter, R.J.; Hermann, D.M.; Bassetti, C.L. Delayed melatonin administration promotes neuronal survival, neurogenesis and motor recovery, and attenuates hyperactivity and anxiety after mild focal cerebral ischemia in mice. J. Pineal. Res. 2008, 45, 142–148.
